| Ils sont nés un 11 octobre |
| Joan Cusack, Gerald Darmanin, Bernard Frank, Jean-Jacques Goldman, Pierre-Jean Jouve, Francois Mauriac |
Lundi 11 octobre :
Demain :
|
| |||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sabian Symbol:
A gang of robbers are seen in hiding, ready and anxious to attack the heavily armed caravan just coming into sight.
Sabian Symbol:
A perspiring fat-boy is mowing the grass; he has discovered the handicap of flesh and is determined to lose it.
Sabian Symbol:
An odd looking collection of machinery parts lie together; all are new and ready to use, all are circular.
Sabian Symbol:
Two men are placed under arrest and taken away to give an accounting for their acts before a tribunal of society.
Sabian Symbol:
A big trained bear sitting on a chair especially built for him is waving all four paws in grotesque fashion.
Sabian Symbol:
In a fantasy presented by the children of an exclusive school a huge egg cracks to reveal a cherubic miss.
Sabian Symbol:
Several children are splashing with delight in a receding tide, and at their feet are shellfish groping for shelter.
Sabian Symbol:
Down the man-made mountain of industry in allegorical representation some the prophet with tablets of a new law.
Sabian Symbol:
The little boys are welcomes to the store of the genial oriental rug dealer for rare fun in piled softness.
Sabian Symbol:
The great artist, a world famous pianist, sits at his instrument on the stage of a huge auditorium.
Sabian Symbol:
A handkerchief of the finest linen and oldest lace lies folded near milady's mirror by a bottle of rare perfume.
Sabian Symbol:
A duck pond is revealed, on which a young brood of ducklings are disporting themselves.
11-Oct-2021, 15:05 UT/GMT | ||||
 | Sun | 18 | 31' 5" | |
 | Moon | 28 | 42'54" | |
 | Mercury | 14 | 21'43"r | |
 | Venus | 4 | 31'47" | |
 | Mars | 17 | 22'43" | |
 | Jupiter | 22 | 24' 8"r | |
 | Saturn | 6 | 52'44" | |
 | Uranus | 13 | 44'51"r | |
 | Neptune | 21 | 4'11"r | |
 | Pluto | 24 | 19'12" | |
 | TrueNode | 2 | 34'12" | |
 | Chiron | 10 | 17'38"r | |
| Planet | Longitude |
| Sun | 18 Lib 31' 06" |
| Moon | 28 Sag 43' 01" |
| Mercury | 14 Lib 21' 43" R |
| Venus | 04 Sag 31' 48" |
| Mars | 17 Lib 22' 43" |
| Jupiter | 22 Aqu 24' 08" R |
| Saturn | 06 Aqu 52' 44" |
| Uranus | 13 Tau 44' 51" R |
| Neptune | 21 Pis 04' 11" R |
| Pluto | 24 Cap 19' 12" |
| Chiron | 10 Ari 17' 38" R |
| Lilith | 09 Gem 26' 37" |
| True Node | 02 Gem 34' 12" |
| Planet | Aspect | Planet | Orb |
| Sun | Conjunction | Mars | 1.14 |
| Sun | Quincunx | Neptune | 2.55 |
| Mercury | Quincunx | Uranus | 0.61 |
| Venus | Sextile | Saturn | 2.35 |
| Venus | Opposition | True Node | 1.96 |
| Mars | Conjunction | Sun | 1.14 |
| Saturn | Sextile | Venus | 2.35 |
| Saturn | Trine | Lilith | 2.56 |
| Uranus | Quincunx | Mercury | 0.61 |
| Neptune | Quincunx | Sun | 2.55 |
| Chiron | Sextile | Lilith | 0.85 |
| Lilith | Trine | Saturn | 2.56 |
| Lilith | Sextile | Chiron | 0.85 |
| True Node | Opposition | Venus | 1.96 |

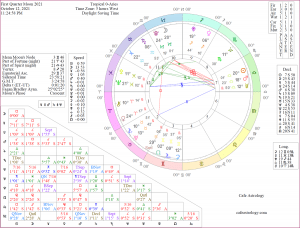
| October 11th, 2021 | |
| The Sun is in Libra | |
| The Moon is in Sagittarius The Crescent Moon is in Sagittarius, Enters Capricorn 19:15 | |
| Current Mercury Retrograde Period Sep 27 - Oct 18 | |

Paris
Mon, Oct 11, 2021
LUNDI11OCTOBRESemaine 41 - jour 285 | FirminFPremier quartier
| Sunrise 08:06 |
| Sunset 19:10 |
| Twilight ends 20:58 begins 06:18 |
30%
5 days old
6th Lunar Day  14:29
14:29
This Lunar Day is propicious to undertaking or carrying out common ventures, well-being, but also lies and cheating.
Waxing Moon
A good time for long-term partnership and for starting the implementation of far-reaching plans.
Moon in Sagittarius 
 19:14
19:14
Suitable energy for travelling, exploring, radiating optimism, engaging in philosophical discussions, speculations, risk taking, gambling, adventures, trekking, camping out, wild life, search for God or Guru. Purchase of travel tickets, camping gear, cars, bikes, horses, dogs, raffle and lottery tickets.
Void of Course Moon ![]()
![]()
![]()
 6:30
6:30  19:14
19:14
Moon's Last Aspect: Sextile Jupiter.
| Rise | Set | |
| Mercury | 07:50 | 18:54 |
| Venus | 12:33 | 20:40 |
| Moon | 14:30 | 22:18 |
| Mars | 08:00 | 19:11 |
| Jupiter | 17:16 | 02:59 |
| Saturn | 16:37 | 01:35 |
Oct 11 2021 17:27 Lun

 12° / 17°
12° / 17°Risque de pluie : 0% - Humi. : 88%
Vent :Nord - 13 km/h
Lever : 08:05 - Coucher : 19:08
Clair
| Planet | Starts | Ends | Planet | Starts | Ends |
|---|---|---|---|---|---|
 | 08:05 | 09:00 |  | 19:11 | 20:15 |
 | 09:00 | 09:56 |  | 20:15 | 21:20 |
 | 09:56 | 10:51 |  | 21:20 | 22:25 |
 | 10:51 | 11:47 |  | 22:25 | 23:29 |
 | 11:47 | 12:42 |  | 23:29 | 00:34 |
 | 12:42 | 13:38 |  | 00:34 | 01:38 |
 | 13:38 | 14:33 |  | 01:38 | 02:43 |
 | 14:33 | 15:29 |  | 02:43 | 03:48 |
 | 15:29 | 16:24 |  | 03:48 | 04:52 |
 | 16:24 | 17:20 |  | 04:52 | 05:57 |
 | 17:20 | 18:15 |  | 05:57 | 07:02 |
 | 18:15 | 19:11 |  | 07:02 | 08:06 |
| Lundi 11 Octobre 2021 17h11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

| October 11, 2021 | Mo |
Re:
| October 12, 2021 | Tu |


- The Moon is in Sagittarius until 19:14, after which the Moon is in Capricorn.
- A void Moon period occurs from 6:30 to 19:14 today.
- The Moon is waxing and in its Waxing Crescent phase.
- The New Moon occurred on October 6th in the sign of Libra, and the First Quarter Moon will happen tomorrow.

Today’s Transits (Eastern Daylight Time)
Date & Time: Oct 11 2021 19:14
Event: Moon enters Capricorn
Description: The Moon in Capricorn
Consolidate. Pay attention to the foundations of life. Repair and restructure. Attend to financial or business matters. Make solid plans and get organized.
Date & Time: Oct 11 2021 6:30
Event: Moon goes void of course
Date & Time: Oct 10 2021 19:30
Event: Tr-Tr Mon Sxt Jup
Description: Transiting Moon Sextile Transiting Jupiter
A great time to take up new feel-good opportunities. Show your confidence and optimism and reap the rewards.
Date & Time: Oct 11 2021 11:02
Event: Tr-Tr Mon SSq Ves
Description: Transiting Moon SemiSquare Transiting Vesta
We can be a little stiff with our emotions, and we can be distracted from our work or focus. There can be difficulty expressing tenderness, concern, or care.
Date & Time: Oct 11 2021 16:08
Event: Tr-Tr Mon Sqq Ura
Description: Transiting Moon SesquiSquare Transiting Uranus
We can easily find ourselves all wound up and nervous. Unpredictable responses from others (and ourselves). Emotional eruptions are possible. Not a good time to make permanent decisions, particularly about relationships and family/domestic matters.
Date & Time: Oct 11 2021 06:14
Event: Tr-Tr Mon Cnj Cap
Description: Transiting Moon Entering Capricorn
Consolidate. Pay attention to the foundations of life. Repair and restructure. Attend to financial or business matters. Make solid plans and get organized.
Date & Time: Oct 11 2021 22:35
Event: Tr-Tr Mon Qnx Nod
Description: Transiting Moon Quincunx Transiting North Node
There may be the need to pause to adjust things before moving plans forward. We could feel at odds with others on an emotional level. Public relations are not as favorable now.
Date & Time: Oct 11 2021 6:32
Event: Tr-Tr Sun Pll Mer
Description: Transiting Sun Parallel Transiting Mercury
Date & Time: Oct 11 2021 17:00
Event: Tr-Tr Mer Pll Pal
Description: Transiting Mercury Parallel Transiting Pallas
Date & Time: Oct 11 2021 17:16
Event: Tr-Tr Mar Cpl Chi
Description: Transiting Mars ContraParallel Transiting Chiron
Date & Time: Oct 11 2021 11:15
Event: Tr-Tr Mar Sqq Nod
Description: Transiting Mars SesquiSquare Transiting North Node
Behavior and desires are at odds with our higher goals. We might take on too much now, or our impatience can interfere with success (haste makes waste). In order to take advantage of new opportunities to achieve our desires, we have to let go of some wilfulness and expectations.
**Conjunctions to Select Fixed Stars on October 11, 2021.**
Aspects to Moon 29°Sg15 -25°45′
conjunct 29°Sg02 ACUMEN Enduring attacks which weaken.
Aspects to Uranus 13°Ta44 +15°33′
conjunct 14°Ta37 MENKAR — A victim of the Unconscious
Aspects to North Node 02°Ge34 +20°40′
conjunct 02°Ge23 MIRFAK Challenge-oriented.
Aspects to Black Moon 09°Ge26 +22°21′
conjunct 10°Ge05 ALDEBARAN *** Success through integrity
THE MOON IN SAGITTARIUS
This is a time for expanding our mind and experience, exploring new pathways, aiming high, and broadening our horizons. It’s not as strong for detail or routine work. There can be restlessness, courage, and spontaneity now.
30TH DEGREE OF SAGITTARIUS
Part of Body: Pear-shaped muscle
Sabian Symbol: The pope blessing the faithful.
THE SUN
THE SUN IN LIBRA
This is the time of year for seeking out more harmony and balance, and paying special attention to our close relationships.
19TH DEGREE OF LIBRA
Part of Body: Calyx major
Sabian Symbol: A gang of robbers in hiding.
ASPECTS OF THE SUN
CONJUNCTION MERCURY Orb 4°14′ Separating
Thoughts and communications about ourselves and our goals. We are expressing ourselves confidently, directly, and clearly.
CONJUNCTION MARS Orb 1°09′ Separating
We are more direct, spontaneous, and impulsive now, but can also be too self-focused and impatient.
TRINE JUPITER Orb 3°50′ Applying
There can be a nice, healthy feeling of optimism and confidence now. We might promote, publish, advertise, or market with greater success. We are generous and tolerant, giving people the benefit of the doubt, expecting the best from others and from ourselves. We can see the big picture.
QUINCUNX NEPTUNE Orb 2°30′ Applying
Dreaming, creating, and imagining have better results than hard decision making right now. There can be a temporary loss/lack of clarity. We can be questoning whether what we are doing or pursuing feeds our spiritual needs. Make adjustments if there is a discrepancy.
SQUARE PLUTO Orb 5°45′ Applying
We may be tempted to control or manipulate events and people, and can have difficulty reaching a compromise. We might need to reevaluate expectations, face our fears, manage power struggles, and deal with urges to control others and situations.
MERCURY
MERCURY IN LIBRA
You are a diplomat and peacemaker, often acting as a go-between in relationships. You a good communicator, putting others at their ease. Arguments may upset you, as you prefer harmony in communications.
15TH DEGREE OF LIBRA
Part of Body: Right inguinal lymph nodes
Sabian Symbol: Circular paths.
ASPECTS OF MERCURY
CONJUNCTION MARS Orb 3°05′ Separating
Questions are raised, discussions can become heated, and there can be nervous excitement now. Enthusiasm is strong, particularly for ideas and topics. Impulsive speech or other communications, and possibly lacking in sensitivity. Resourcefulness.
QUINCUNX URANUS Orb 0°34′ Applying
Things may not be running smoothly, and we may need to make adjustments to accommodate others’ input or changes of plans or opinions.
OPPOSITION CHIRON Orb 4°01′ Applying
There can be insecurity about our own ideas or decisions, or we may not know who/what to believe. Words, or lack of, can hurt right now. There is greater sensitivity to what is being said, choice of words, and syntax.
VENUS
VENUS IN SAGITTARIUS
You want to share adventure with your partner, ranging from sharing adventurous ideas to hiking in the mountains. You may also enjoy relating with foreigners, and dining out with your partner at foreign restaurants.
5TH DEGREE OF SAGITTARIUS
Part of Body: Right femoral artery
Sabian Symbol: An old owl up in a tree.
ASPECTS OF VENUS
SEXTILE SATURN Orb 2°18′ Applying
We are conservative with our feelings, affections, and pleasures. We value loyalty, duty, honor, steadiness, and economy now, and our judgment is sound if on the cautious side. Smart purchases. Maturity in love and with money and business. A good time to form personal or professional alliances.
TRINE CHIRON Orb 5°43′ Applying
Opportunities for relationships or socializing can arise now. Interactions are open, warm, and supportive of growth.
OPPOSITION THE NORTH NODE Orb 2°00′ Separating
We may be re-evaluating relationships in terms of whether or not they are contributing to our personal growth. There could be some superficiality in our interactions now.
CONJUNCTION THE SOUTH NODE Orb 2°00′ Separating
We may be re-evaluating relationships in terms of whether or not they are contributing to our personal growth. There could be some superficiality in our interactions now.
MARS
MARS IN LIBRA
You have a strong desire for diplomacy and justice, and a strong creative urge. You may become indecisive when opposed.
18TH DEGREE OF LIBRA
Part of Body: Fatty capsule of kidneys
Sabian Symbol: Two men placed under arrest.
ASPECTS OF MARS
TRINE JUPITER Orb 4°59′ Applying
You are enthusiastic with an endless source of energy. You could direct your energy to achievement in sports, politics or travel.
JUPITER
JUPITER IN AQUARIUS
You are a seeker of the new and inspirational. You hold racially-inclusive values, and are a humanitarian.
23RD DEGREE OF AQUARIUS
Part of Body: Left gastroscnemius muscle
Sabian Symbol: A big bear sitting down and waving all its paws.
SATURN
SATURN IN AQUARIUS
You have an ability to work well in groups. Your work is often original and innovative.
7TH DEGREE OF AQUARIUS
Part of Body: Right saphenous veins
Sabian Symbol: A child born of an eggshell.
ASPECTS OF SATURN
SEXTILE CHIRON Orb 3°24′ Applying
You have the opportunity through perseverance to face emotional pain in your life and discover your own natural sense of authority.
URANUS
URANUS IN TAURUS
(1934 – 1942) We approach money and personal possessions in new ways and learn how to free ourselves from certain material constraints. Innovative ways to make ourselves comfortable emerge. We’re less inhibited about expressing sensuality, self-love, body love, and pampering. We’re challenging what we previously valued. There can be abrupt changes with money, valuables, possessions, and income that lead to a reshuffling of priorities or values. Income might come from non-traditional sources or ventures. We’re bringing progressive ideas to the world of business. New ways of doing business, as well as making, viewing, and handling money are likely. Income and the energy we put into making money can be variable. (May 15, 2018, to November 6, 2018, then March 6, 2019, to July 7, 2025, and then November 7, 2025, to April 25, 2026).
14TH DEGREE OF TAURUS
Part of Body: True vocal cords
Sabian Symbol: Shellfish groping and children playing.
NEPTUNE
NEPTUNE IN PISCES
A long-term influence in which fantasy, imagination, compassion, and spirituality are in stronger focus. (April 4, 2011, to August 4, 2011, then February 3, 2012, to March 30, 2025, then October 22, 2025, to January 26, 2026)
22ND DEGREE OF PISCES
Part of Body: Achilles tendon of the right foot
Sabian Symbol: A man bringing down the new law from Sinai.
PLUTO
PLUTO IN CAPRICORN
Tests of our boundaries; breaking down and rebuilding structures and rules. (From January 25, 2008, to June 14, 2008, then November 26, 2008, to March 23, 2023, then June 11, 2023, to January 20, 2024, then September 1, 2024, to November 19, 2024).
25TH DEGREE OF CAPRICORN
Part of Body: Connections between femur and tibia
Sabian Symbol: An oriental rug dealer.

First Quarter Moon
The FIRST QUARTER MOON occurs on Tuesday, October 12th, 2021, at 11:25 PM EDT.
On Tuesday night, the First Quarter Moon is exact, when the Sun in Libra forms a square with the Moon in Capricorn.
There is a crisis theme surrounding any quarter Moon phase, as we feel compelled to take action. Shortly after, we are made aware of how our actions affect those close to us, perhaps through trial and error. Whatever project or initiative we began around the New Moon is now off the ground, and it may face its first obstacles.
This phase of the Moon occurs at 20 degrees and 1 minute of Capricorn square the Sun at 20 degrees and 1 minute of Libra, affecting people born with personal planets and points at approximately 18 to 22 degrees of the Cardinal signs (Aries, Cancer, Libra, and Capricorn) most significantly. This chart wheel includes extra points like Chiron, the four major asteroids, Eris, and Sedna.
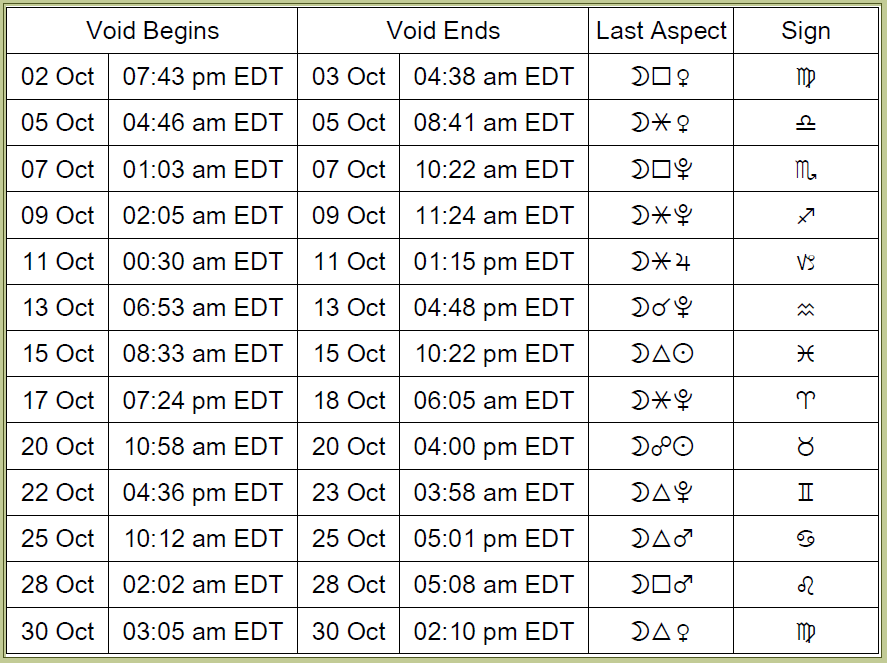
October 2021:
Timing with the Moon – Better Periods this Week for Electing New Initiatives
Void-of-course Moon on Monday, October 11th, from 12:30 AM EDT, with the Moon’s last aspect before changing signs (a sextile to Jupiter), until the Moon enters Capricorn at 1:15 PM EDT.
VOC Moon on Wednesday, October 13th, from 6:53 AM EDT, with the Moon’s last aspect before changing signs (a conjunction to Pluto), until the Moon enters Aquarius at 4:48 PM EDT.
VOC Moon on Friday, October 15th, from 8:33 AM EDT, with the Moon’s last aspect before changing signs (a trine to the Sun), until the Moon enters Pisces at 10:22 PM EDT.
The Moon is waxing this week until the New Moon on the 6th, which is usually considered favorable for new beginnings, with exceptions. (See Best Time to Start a Business for more details).
It’s important to note that there are more factors to consider than only the below windows. For example, you generally don’t want to elect a new venture when a very challenging aspect is applying, and it’s preferable to elect a time when a particularly positive aspect hasn’t yet perfected (is applying). The following windows are starting points.
According to principles of timing with the Moon, a short window of opportunity occurs this week from Sunday, October 10th, at approximately 11:20 PM EDT to Monday, October 11th, at approximately 12:25 AM EDT, while the Moon is in Sagittarius. Another window occurs on Wednesday, October 13th, from approximately 12:30 AM to 6:50 AM EDT, while the Moon is in Capricorn.
A better window occurs from Thursday, October 14th, at approximately 5:55 PM EDT, to Friday, October 15th, at approximately 8:30 AM EDT, while the Moon is in Aquarius. This one is better before the Sun and Jupiter perfect their trine–in other words, until 7:45 AM on October 15th.
Another window occurs from Saturday, October 16th, from approximately 6:00 PM EDT, until Sunday, October 17th, at approximately 7:20 PM, while the Moon is in Pisces.
Caveats: note that Mercury is retrograde during these windows. Mercury’s direct station occurs on October 18th.
OCTOBER 11
The Moon continues its transit of spirited Sagittarius until 1:15 PM, after which it’s in Capricorn. The Capricorn Moon inspires a more orderly, practical approach to our day. However, Mercury is heading into a quincunx with Uranus. This transit can complicate our interactions as the day advances. It can be challenging to get a point across clearly or even kindly. We should watch for poorly-considered decisions, impulsive communications, and disorganization leading to misunderstandings.
The void Moon occurs from 12:30 AM EDT, with the Moon’s last aspect before changing signs (a sextile to Jupiter), until the Moon enters Capricorn at 1:15 PM EDT.
OCTOBER 12
The Moon continues its transit of Capricorn all day, and the First Quarter Moon is exact tonight when the Capricorn Moon forms a square to the Sun in Libra. There is a crisis theme surrounding any quarter Moon phase, as we feel compelled to take action. Shortly after, we become aware of how our actions affect those close to us, perhaps through trial and error. It’s a time of stress, but it’s the kind of tension that motivates us to make changes. Relationship and material goals tend to clash, causing some pressure or anxiety. Even so, transits today help us find a balance between learning from tradition and seeing things progressively.
 |
Sun enters the next Sabian symbol 11/10 at 04:31 h (chart) |
 |
Sun enters the next Sabian symbol 12/10 at 04:47 h (chart) |
Sun in Libra Meeting yourself in another person is the keynote of a new cycle which begins for you now. Relationships – romantic, business, social – are the arena where this drama is played out. In coming to know the other person, forging ties that bind two people, you come to a better understanding of yourself. It’s easy in theory, but it’s only in practice that it comes to mean anything real – so practice until you get it right!
Mercury in Libra A new cycle begins for you, signaling a greater than usual interest in relationships, social connections, and the arts – on a more intellectual level than in the past, most likely. Seeing both sides of an issue, and figuring out resolutions to opposing views: these things take on more importance in your life.
Venus in Sagittarius A yearning for adventure and far horizons stirs in you now. To roam, to wander and wonder, to seek freedom and go where no one has gone before – these things are deep and powerful longings. A time of romantic crusades, a universal love that might not be too particular.
Mars in Libra “I’m giving you a definite maybe” – that’s the motto of the cycle you’re now entering. It’s a time of refinement and tact, of an urge to please others that is so strong as to make it hard to decide or act for fear of offending someone. You’ll quickly learn to balance this out, or you’ll discover that there is no peace in compromise – and this can be tough on close relationships. The trick is to find the win-win solution to the no-win situation – and if anyone’s ever up to it, you are now.
Jupiter in Aquarius Innovation, reform, and idealism are your paths to growth and gain now. New, futuristic ideas are part of this, but it’s much more than that: putting them into practice is essential because this is much more than empty idealism. A break from the past, a willingness to flaunt convention.
Saturn in Aquarius Ideals are in for a period of testing, as a new phase begins in your life. What are principles worth, unless they are put into practice? That’s an important question for you now. This could mean you may be disappointed by causes you believe in – but if you abandon your principles, you lose.
Uranus in Taurus Practicality and ingenuity combine as powerful drives in your life now that you have begun a new cycle. Taking innovative concepts and making them real and practical is your strong suit; getting stuck in the mud is to be avoided at all costs. Financial savvy, inventiveness, worldly genius.
Neptune in Pisces Things spiritual and psychic take the driver’s seat of your imagination as you begin a new cycle. The fine arts and all manner of fantasies assume a larger-than-life importance. Surrendering to fate, karma, and other powers greater than merely mortal may be seen as the ultimate liberation. If all is Maya (illusion), what is the reality that gives rise to the world of appearances?
Pluto in Capricorn As this new phase dawns for you, the pursuit of social/political power and status has a way of seeming necessary and inevitable – and let nothing stand in its way. It starts out small but has bigger consequences than you’d think. Ambition, responsibility, a place for everyone, and everyone in their place…
- The Moon is in Sagittarius.
- There is no void Moon period today.
- The Moon is waxing and in its Waxing Crescent phase.
- The New Moon occurred on October 6th in the sign of Libra.

Oct 6, 2021, 7:05 AM, Sun Conjunct Moon (New Moon)
Oct 12, 2021, 11:25 PM, Sun Square Moon (First Quarter Moon)
Oct 20, 2021, 10:57 AM, Sun Opposition Moon (Full Moon)
Oct 28, 2021, 4:05 PM, Sun Square Moon (Last Quarter Moon)
Nov 4, 2021, 5:14 PM, Sun Conjunct Moon (New Moon)

![]() Venus in Sagittarius A yearning for adventure and far horizons stirs in you now. To roam, to wander and wonder, to seek freedom and go where no one has gone before – these things are deep and powerful longings. A time of romantic crusades, a universal love that might not be too particular.
Venus in Sagittarius A yearning for adventure and far horizons stirs in you now. To roam, to wander and wonder, to seek freedom and go where no one has gone before – these things are deep and powerful longings. A time of romantic crusades, a universal love that might not be too particular.
Venus parallel Pluto October 7. The desire for drama and intensity in relationships is with us. Love feelings and relationship issues can be all-consuming now, assuming more importance in our lives than usual. Interactions with others are intense now, and themes of sharing and trust dominate in relationships.
Venus semi-square Mercury October 8-9. Communications between lovers are superficial and unsatisfying, and perhaps awkward. Superficiality in romantic expression may exist now.
Venus opposition North Node October 9-10. This is a time for reviewing our attachments in terms of whether they are contributing to our growth.
Venus sextile Saturn October 13. Relationships are stabilized and steady, although feelings are expressed reservedly, formally, or sparingly. A new sense of realism in existing partnerships. Relationships begun under this influence possess a distinctly practical theme, almost as if the partnership is a business endeavor.
Venus semi-square Pluto October 15. Tendencies to look for something wrong in a relationship. Jealousy raises its ugly head.
Venus sextile Mercury October 16. Favors playfulness in love and enhanced communication between partners. Romantic overtures, expressions of love and affection, making new social contacts. This is a time when promises, pledges, and clarifications (usually very positive!) are made in love relationships.
Venus trine Chiron October 16. A commitment-friendly transit. Love can heal old wounds. We are open to romantic feelings.
Venus quincunx (inconjunct) Uranus October 19. Impulsive spending, peculiar attractions, fear of commitment, freedom vs. intimacy issues. We might question whether we need more excitement in our relationships, or whether we need freedom from them; yet we don’t feel confident enough to rock the boat.
Venus square Neptune October 26. Relationship ups and downs characterize this transit–a time when romantic mirages are more than likely. We see what we hope to see, rather than what really is. The “highs” of a relationship begun under this energy may be thrilling, but the accompanying “lows” are bound to set in, and they can be extremely draining. Be careful not to set yourself up for disappointment.
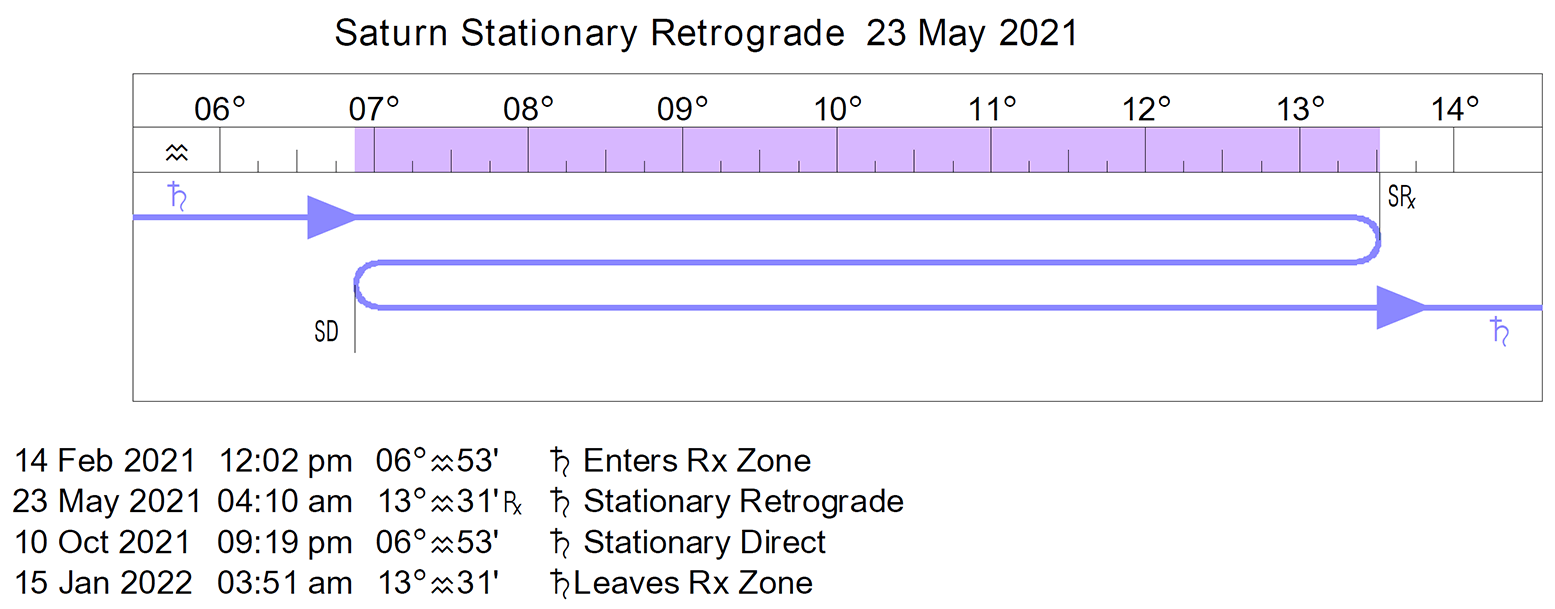
- Saturn stations and turns direct today (Saturn was retrograde from May 23 to October 10th).
- Mercury is retrograde (Mercury is retrograde from September 27 to October 18th).
- Mercury Rx, Jupiter Rx, Uranus Rx, Neptune Rx, and Chiron Rx.
Saturn now turns retrograde at 06° Aquarius 53′.
Saturn turned retrograde on May 23, 2021, at 13° Aquarius 31′ Saturn turns direct on October 10, 2021, at 06° Aquarius 53′
Sun in Libra Meeting yourself in another person is the keynote of a new cycle which begins for you now. Relationships – romantic, business, social – are the arena where this drama is played out. In coming to know the other person, forging ties that bind two people, you come to a better understanding of yourself. It’s easy in theory, but it’s only in practice that it comes to mean anything real – so practice until you get it right!
Mercury in Libra A new cycle begins for you, signaling a greater than usual interest in relationships, social connections, and the arts – on a more intellectual level than in the past, most likely. Seeing both sides of an issue, and figuring out resolutions to opposing views: these things take on more importance in your life.
Venus in Sagittarius A yearning for adventure and far horizons stirs in you now. To roam, to wander and wonder, to seek freedom and go where no one has gone before – these things are deep and powerful longings. A time of romantic crusades, a universal love that might not be too particular.
Mars in Libra “I’m giving you a definite maybe” – that’s the motto of the cycle you’re now entering. It’s a time of refinement and tact, of an urge to please others that is so strong as to make it hard to decide or act for fear of offending someone. You’ll quickly learn to balance this out, or you’ll discover that there is no peace in compromise – and this can be tough on close relationships. The trick is to find the win-win solution to the no-win situation – and if anyone’s ever up to it, you are now.
Jupiter in Aquarius Innovation, reform, and idealism are your paths to growth and gain now. New, futuristic ideas are part of this, but it’s much more than that: putting them into practice is essential because this is much more than empty idealism. A break from the past, a willingness to flaunt convention.
Saturn in Aquarius Ideals are in for a period of testing, as a new phase begins in your life. What are principles worth, unless they are put into practice? That’s an important question for you now. This could mean you may be disappointed by causes you believe in – but if you abandon your principles, you lose.
Uranus in Taurus Practicality and ingenuity combine as powerful drives in your life now that you have begun a new cycle. Taking innovative concepts and making them real and practical is your strong suit; getting stuck in the mud is to be avoided at all costs. Financial savvy, inventiveness, worldly genius.
Neptune in Pisces Things spiritual and psychic take the driver’s seat of your imagination as you begin a new cycle. The fine arts and all manner of fantasies assume a larger-than-life importance. Surrendering to fate, karma, and other powers greater than merely mortal may be seen as the ultimate liberation. If all is Maya (illusion), what is the reality that gives rise to the world of appearances?
Pluto in Capricorn As this new phase dawns for you, the pursuit of social/political power and status has a way of seeming necessary and inevitable – and let nothing stand in its way. It starts out small but has bigger consequences than you’d think. Ambition, responsibility, a place for everyone, and everyone in their place…

| DateOct 2021 | Moon Aspects | Chart | |||
| Oct 1, 00:54 | chart | ||||
| Oct 1, 14:09 | chart | ||||
| Oct 1, 17:33 | chart | ||||
| Oct 1, 23:45 | chart | ||||
| Oct 2, 05:31 | chart | ||||
| Oct 2, 21:28 | chart | ||||
| Oct 2, 22:56 | chart | ||||
| Oct 3, 01:42 | chart | ||||
| Oct 3, 10:38 | chart | ||||
| Oct 4, 11:18 | chart | ||||
| Oct 4, 23:47 | chart | ||||
| Oct 5, 05:02 | chart | ||||
| Oct 5, 10:45 | chart | ||||
| Oct 5, 16:41 | chart | ||||
| Oct 6, 02:15 | chart | ||||
| Oct 6, 13:05 | chart | ||||
| Oct 6, 14:03 | chart | ||||
| Oct 6, 23:39 | chart | ||||
| Oct 7, 04:07 | chart | ||||
| Oct 7, 07:02 | chart | ||||
| Oct 7, 16:22 | chart | ||||
| Oct 8, 03:37 | chart | ||||
| Oct 8, 14:59 | chart | ||||
| Oct 9, 02:52 | chart | ||||
| Oct 9, 05:03 | chart | ||||
| Oct 9, 08:05 | chart | ||||
| Oct 9, 17:24 | chart | ||||
| Oct 9, 21:36 | chart | ||||
| Oct 10, 04:42 | chart | ||||
| Oct 10, 18:48 | chart | ||||
| Oct 10, 21:11 | chart | ||||
| Oct 10, 22:44 | chart | ||||
| Oct 11, 04:16 | chart | ||||
| Oct 11, 06:30 | chart | ||||
| Oct 11, 19:15 | chart | ||||
| Oct 12, 17:50 | chart | ||||
| Oct 12, 18:33 | chart | ||||
| Oct 13, 02:26 | chart | ||||
| Oct 13, 05:24 | chart | ||||
| Oct 13, 07:10 | chart | ||||
| Oct 13, 12:53 | chart | ||||
| Oct 13, 22:48 | chart | ||||
| Oct 14, 10:54 | chart | ||||
| Oct 14, 12:03 | chart | ||||
| Oct 14, 19:08 | chart | ||||
| Oct 14, 22:51 | chart | ||||
| Oct 15, 09:57 | chart | ||||
| Oct 15, 14:28 | chart | ||||
| Oct 15, 14:32 | chart | ||||
| Oct 16, 04:22 | chart | ||||
| Oct 16, 22:59 | chart | ||||
| Oct 17, 05:14 | chart | ||||
| Oct 17, 18:59 | chart | ||||
| Oct 18, 01:23 | chart | ||||
| Oct 18, 12:04 | chart | ||||
| Oct 19, 01:14 | chart | ||||
| Oct 19, 07:25 | chart | ||||
| Oct 19, 12:39 | chart | ||||
| Oct 20, 06:58 | chart | ||||
| Oct 20, 08:28 | chart | ||||
| Oct 20, 10:55 | chart | ||||
| Oct 20, 16:56 | chart | ||||
| Oct 20, 21:59 | chart | ||||
| Oct 21, 11:44 | chart | ||||
| Oct 22, 00:27 | chart | ||||
| Oct 22, 15:26 | chart | ||||
| Oct 22, 18:31 | chart | ||||
| Oct 22, 22:35 | chart | ||||
| Oct 23, 09:57 | chart | ||||
| Oct 24, 00:11 | chart | ||||
| Oct 24, 12:16 | chart | ||||
| Oct 24, 23:41 | chart | ||||
| Oct 25, 04:13 | chart | ||||
| Oct 25, 07:33 | chart | ||||
| Oct 25, 16:10 | chart | ||||
| Oct 25, 23:00 | chart | ||||
| Oct 26, 04:54 | chart | ||||
| Oct 27, 01:36 | chart | ||||
| Oct 27, 07:09 | chart | ||||
| Oct 27, 16:47 | chart | ||||
| Oct 28, 00:07 | chart | ||||
| Oct 28, 08:01 | chart | ||||
| Oct 28, 11:08 | chart | ||||
| Oct 28, 22:05 | chart | ||||
| Oct 29, 01:02 | chart | ||||
| Oct 29, 12:24 | chart | ||||
| Oct 30, 00:31 | chart | ||||
| Oct 30, 06:23 | chart | ||||
| Oct 30, 09:04 | chart | ||||
| Oct 30, 20:10 | chart | ||||
| Oct 30, 20:21 | chart | ||||
| Oct 31, 10:57 | chart | ||||
| Oct 31, 19:32 | chart | ||||




| Date | Moon Aspects and Ingresses | Chart | |
| Oct 1, 02:53 | Enters 0° Leo | chart | |
| Oct 3, 10:37 | Enters 0° Virgo | chart | |
| Oct 5, 14:40 | Enters 0° Libra | chart | |
| Oct 6, 13:05 | chart | ||
| Oct 6, 14:03 | chart | ||
| Oct 6, 23:39 | chart | ||
| Oct 7, 16:21 | Enters 0° Scorpio | chart | |
| Oct 9, 17:23 | Enters 0° Sagittarius | chart | |
| Oct 9, 21:36 | chart | ||
| Oct 11, 19:14 | Enters 0° Capricorn | chart | |
| Oct 13, 12:53 | chart | ||
| Oct 13, 22:47 | Enters 0° Aquarius | chart | |
| Oct 14, 10:54 | chart | ||
| Oct 15, 14:28 | chart | ||
| Oct 16, 04:21 | Enters 0° Pisces | chart | |
| Oct 17, 18:59 | chart | ||
| Oct 18, 12:03 | Enters 0° Aries | chart | |
| Oct 20, 16:56 | chart | ||
| Oct 20, 21:58 | Enters 0° Taurus | chart | |
| Oct 22, 00:27 | chart | ||
| Oct 23, 09:56 | Enters 0° Gemini | chart | |
| Oct 25, 22:59 | Enters 0° Cancer | chart | |
| Oct 28, 11:07 | Enters 0° Leo | chart | |
| Oct 30, 20:09 | Enters 0° Virgo | chart | |


Vivre avec la Lune c’est un peu comme se laisser porter par le courant…
Nos aïeux adaptaient naturellement leur rythme de vie à celui de la Lune. En ces temps là, leur proximité avec la nature faisait qu’une connexion s’établissait entre les rythmes du corps et les rythmes lunaires. De plus, la nécessité de prendre en compte les influences lunaires afin d’optimiser certaines actions du quotidien poussait les gens à observer les cycles lunaires. En effet, pour obtenir une bonne récolte par exemple, il fallait obligatoirement prendre en compte tous les paramètres qui pouvaient favoriser le rendement. Les influences lunaires étaient donc considérées avec une grande attention; d’autant plus que les anciens à force d’observations avaient déjà mis en évidence l’influence que pouvait avoir les astres sur la terre et sur leur quotidien.
Depuis cette époque, les moyens mécaniques ont considérablement réduit les efforts à fournir pour se nourrir et notre dépendance à la nature a diminué petit à petit. Nous nous sommes ainsi éloignés d’un mode de vie en harmonie avec les cycles lunaires, pour définir un rythme de vie qui nous est propre. Malheureusement pour nous, ce rythme ne va pas toujours dans le sens de la logique ni du naturel.
De nos jours, on constate d’ailleurs de plus en plus une envie, voire un besoin de retrouver un équilibre avec les forces de la nature. La question de l’efficacité alliée à une pratique naturelle est la raison prépondérante quant au choix d’accorder son quotidien aux rythmes lunaires. D’ailleurs, nombreux sont ceux qui aujourd’hui ont pu constater une efficacité dans divers domaines tels que la coupe des cheveux, l’épilation, les soins du corps et bien évidemment le jardinage, il existe d’autres nombreuses applications en relation avec la Lune…
Dans le Calendrier Lunaire nous ne proposons pas seulement des conseils mais aussi et surtout des dates pour optimiser vos actions selon que vous recherchiez le meilleur moment pour couper et ainsi vitaliser votre chevelure (la rendre plus épaisse ou en ralentir la chute, etc.), le meilleur moment pour s’épiler afin que la repousse du poil soit plus lente ou encore une bonne date pour des soins ( peau, ongles) ou traitements de verrues, cors, durillons, etc. Vous y trouverez également des conseils sur l’alimentation. De plus vous pourrez définir les dates ou votre corps réagira le mieux à certains traitements: soins, massages, intervention dentaire ou chirurgicale… En effet, suivant les phases lunaires et les aspects planétaires votre corps réagira plus ou moins bien à l’intervention et réduira ou augmentera le temps de convalescence (il va de soi qu’il s’agit là d’interventions programmables et à caractère non-urgent).
Suivant les phases lunaires, l’énergie physique sera donc stimulée ou diminuée. Par exemple, on pourra stimuler un organe en lune croissante tandis qu’on éliminera les toxines d’un organe avec beaucoup plus de succès en lune décroissante. La lune croissante sera une meilleure période pour démarrer un projet alors que la lune décroissante sera plutôt une période propice à l’intériorisation ou la réflexion….
Bien sûr tout le monde ne réagit pas de la même manière aux influences lunaires, qui se font ressentir plus ou moins selon l’âge, la vitalité ou la sensibilité. Au moment de la pleine lune par exemple, certaines personnes sont perturbées dans leur sommeil alors que d’autres ne ressentent rien. Pourtant, une étude réalisée par des chercheurs suisses et publiée dans la revue “Current Biology” a montré qu’au moment de la pleine lune les taux de mélatonine diminuent, d’où une baisse de 30% du sommeil profond. La vitalité et l’excitabilité sont donc plus importantes au moment de la pleine lune, on le remarque d’ailleurs facilement chez les enfants.
http://www.cell.com/current-biology/retrieve/pii/S0960982213007549
En conclusion, même si nous ne percevons pas tous de la même manière les influences lunaires, elles existent et leur efficacité est de plus en plus reconnue.

Merck seeks US authorization for Covid treatment pill
Oct 11 2021

Capsules of the experimental anti-Covid drug molnupiravir, for which Merck is seeking emergency use authorization in the US
Washington (AFP) - US pharmaceutical giant Merck on Monday applied for emergency use authorization of its oral anti-Covid drug in the United States, a major step towards finding a simple pill to treat the disease.
Merck has submitted the application for molnupiravir, which it said earlier this month was shown to reduce hospitalizations by 50 percent.
It also prevented 100 percent of deaths compared to a placebo, but the sample size was relatively small and the figure can’t yet be reliably extrapolated.
Merck, which is called MSD outside the United States and Canada, said it was working “with regulatory agencies worldwide to submit applications for emergency use or marketing authorization in the coming months.”
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency,” Robert Davis, the company’s chief executive officer and president said in the statement.
“That is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” he added.
In a late stage clinical trial, Merck and its partner Ridgeback Biotherapeutics evaluated data from around 770 patients – roughly half of whom received either a five-day course of the pill, while the other received a placebo.
All the patients had lab-confirmed Covid-19 with symptoms that developed within five days of them being assigned to their respective groups.
Of the patients who received molnupiravir, 7.3 percent were hospitalized by day 29, compared to 14.1 percent of those on a placebo – a relative risk reduction of around 50 percent.
Importantly, no deaths were reported in patients who received molnupiravir, as compared to eight deaths in patients who received placebo.
Efficacy was said to hold up against variants of concern, including Delta, and the drug had a good safety profile.
Merck said in the statement that it expects to produce 10 million five-day-courses of treatment by the end of 2021, with more courses expected to be produced in 2022.
The US has procured 1.7 million courses of molnupiravir should it be approved, with the option to buy more, and global health agency Unitaid has said it was working with a partnership called ACT-Accelerator to secure supply for low- and middle-income countries.
Merck said it “plans to implement a tiered pricing approach based on World Bank country income criteria to reflect countries’ relative ability to finance their health response to the pandemic.”
The company said that, pending authorizations, it has also signed non-exclusive voluntary licensing agreements for molnupiravir with established Indian generic manufacturers to accelerate the drug’s availability in more than 100 low- and middle-income countries.
- No miracle cure -
A simple pill to treat the coronavirus has been sought since the start of the pandemic and Friday’s announcement was hailed as a major step towards that goal.
Until now, Covid therapeutics such as monoclonal antibodies and Gilead’s remdesivir have been administered intravenously.
But experts have said it is not a miracle cure and should complement vaccines, not replace them.
They have also cautioned it would be critical to administer the drug early for it to be effective.
Since it isn’t always clear who is at risk for developing severe disease, it would have the greatest impact if it were cheap enough and safe enough to distribute widely.
Molnupiravir belongs to a class of antiviral drugs called polymerase inhibitors, which work by targeting an enzyme that viruses need to copy their genetic material, and introducing mutations that leave them unable to replicate – known as “error catastrophe.”
Such drugs are expected to be more variant-proof than monoclonal antibody treatments, which target a surface protein of the virus that is continually evolving.
Molnupiravir was initially developed as an inhibitor of influenza and respiratory syncytial virus, two other important acute respiratory infections, by a team at Emory University.
Agence France-Presse
AFP journalists cover wars, conflicts, politics, science, health, the environment, technology, fashion, entertainment, the offbeat, sports and a whole lot more in text, photographs, video, graphics and online.
© 2021 AFP


 1:13
1:13 2:53
2:53 19:13
19:13 2:23
2:23 19:06
19:06 3:37
3:37 10:37
10:37 18:16
18:16 4:54
4:54 16:45
16:45 6:13
6:13 14:40
14:40 14:40
14:40 7:34
7:34 12:09
12:09 13:05
13:05 13:05
13:05 8:56
8:56 9:20
9:20 16:21
16:21 6:22
6:22 10:21
10:21 19:29
19:29 3:21
3:21 11:47
11:47 17:23
17:23 0:26
0:26 13:11
13:11 15:35
15:35 17:05
17:05 5:25
5:25 15:18
15:18 16:26
16:26 22:47
22:47 13:52
13:52 17:05
17:05 12:50
12:50 17:34
17:34 4:21
4:21 12:11
12:11 17:56
17:56 11:55
11:55 18:15
18:15 12:03
12:03 12:03
12:03 18:32
18:32 12:36
12:36 18:48
18:48 13:34
13:34 16:56
16:56 19:04
19:04 21:58
21:58 14:55
14:55 19:21
19:21 16:38
16:38 19:41
19:41 9:57
9:57 18:38
18:38 20:05
20:05 17:27
17:27 20:35
20:35 20:48
20:48 21:13
21:13 23:00
23:00 23:00
23:00 22:01
22:01 1:01
1:01 22:58
22:58 2:40
2:40 22:05
22:05 0:03
0:03 1:14
1:14