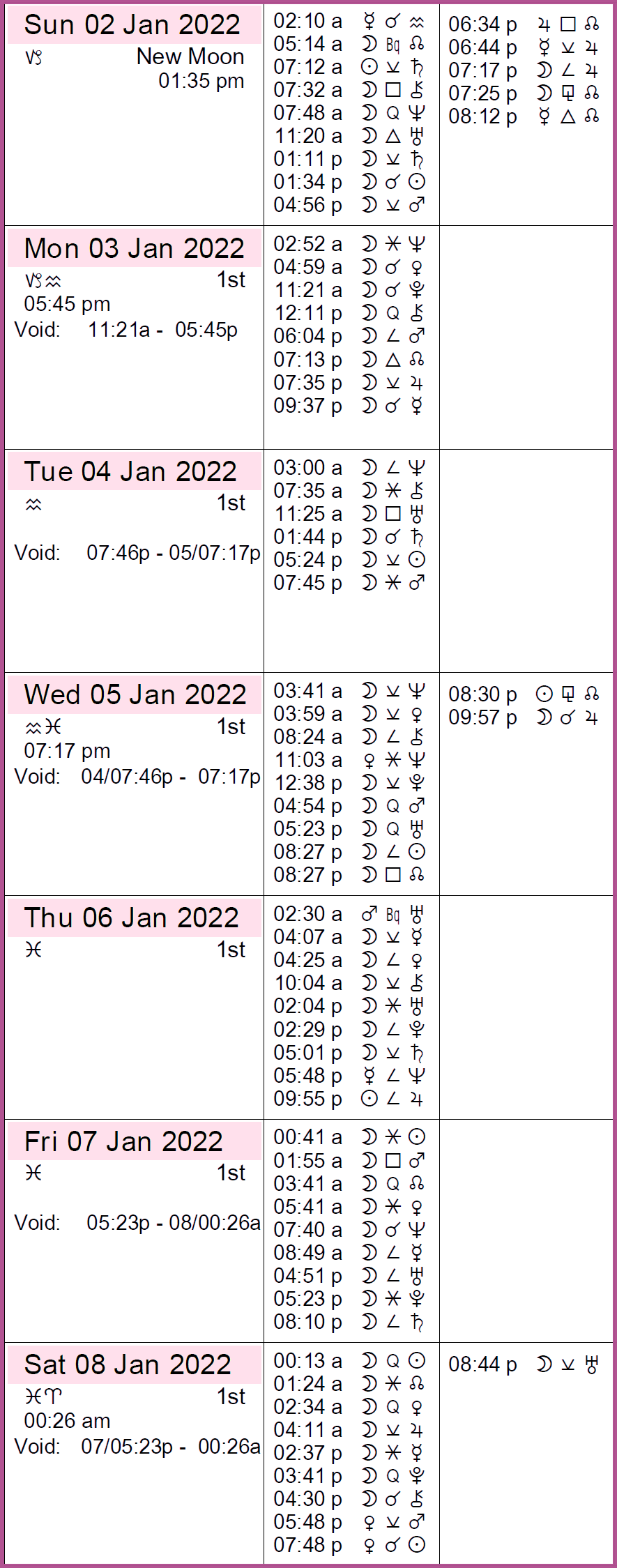
| Ils sont nés un 5 janvier |
| Konrad Adenauer, Ali Bhutto, Alfred Brendel, Bradley Cooper, Umberto Eco, Diane Keaton, Jacques Laurent, Marilyn Manson, Dany Saval, Marc yu, Juan Carlos I. |
- Manson, Marilyn
- Juan Carlos I, King of Spain
- Yogananda, Paramahansa
- Padukone, Deepika
- Duvall, Robert
- Eco, Umberto
- Ailey, Alvin
- Cooper, Bradley
- Mondale, Walter
- Keaton, Diane
- Bartlett, Man
- Somoza Debayle, Anastasio
- Guerritore, Monica
- Ildefonso, Miguel
- Rumpf, Friedrich Karl Georg
- Tanguy, Yves
- Olivo, America
- Adenauer, Konrad
- Watson, Pete
- Mizrachi, Michael
Demain :
|
| |||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
16th Degree of Capricorn
Sabian Symbol:
The high school grounds are alive with fresh vitality as the boys appear in their gymnasium suits.
25th Degree of Aquarius
Sabian Symbol:
A butterfly struggles to emerge from the chrysalis and it seems that the right wing is more perfectly formed.
5th Degree of Aquarius
Sabian Symbol:
In the land of shades a grave council of the ancestors of a man of world importance has been called to guide him.
21st Degree of Capricorn
Sabian Symbol:
Excitement thrills the grandstands during the relay race as each runner springs to place with eagerness.
17th Degree of Sagittarius
Sabian Symbol:
An Easter sunrise service is abut to begin at the suggestion of dawn; darkness surrenders to the worshipers.
2nd Degree of Pisces
Sabian Symbol:
Almost as if possessed with man's intelligence a tiny squirrel remains watchful on a limb hidden from the hunter's.
13th Degree of Aquarius
Sabian Symbol:
Under the shade of the porch of an old-fashioned hotel in a happy little village hangs a sedate barometer.
11th Degree of Taurus
Sabian Symbol:
A woman, cool in sunbonnet and simple garments, is leisurely watering long rows of flowers in full bloom.
21st Degree of Pisces
Sabian Symbol:
A child who is strange to rural life has taken violent fancy to a little white lamb and a Chinese servant smiles.
27th Degree of Capricorn
Sabian Symbol:
A party of anchorites are making a mountain pilgrimage and in view lie both the busy world and the quiet way ahead.
18th Degree of Pisces
Sabian Symbol:
The celebrated revivalist has erected his huge tent, now warmed by music, lights and the smell of sawdust.
18th Degree of Scorpio
Sabian Symbol:
A winding quiet road, carpeted by the falling leaves, leads through an old fashioned woods rich in autumn color.
20th Degree of Capricorn
Sabian Symbol:
There is no service in the church but rising full and clear come the voices of a hidden choir in rehearsal.
5-Jan-2022, 14:32 UT/GMT | ||||
 | Sun | 15 | 13'30" | |
 | Moon | 24 | 14'38" | |
 | Mercury | 4 | 15'33" | |
 | Venus | 20 | 47'34"r | |
 | Mars | 16 | 22'48" | |
 | Jupiter | 1 | 27'44" | |
 | Saturn | 12 | 24'29" | |
 | Uranus | 10 | 53'32"r | |
 | Neptune | 20 | 45'15" | |
 | Pluto | 26 | 5' 1" | |
 | TrueNode | 0 | 47'14"r | |
 | Chiron | 8 | 34' 4" | |
| Planet | Longitude |
| Sun | 15 Cap 13' 31" |
| Moon | 24 Aqu 14' 45" |
| Mercury | 04 Aqu 15' 34" |
| Venus | 20 Cap 47' 34" R |
| Mars | 16 Sag 22' 48" |
| Jupiter | 01 Pis 27' 44" |
| Saturn | 12 Aqu 24' 29" |
| Uranus | 10 Tau 53' 32" R |
| Neptune | 20 Pis 45' 15" |
| Pluto | 26 Cap 05' 01" |
| Chiron | 08 Ari 34' 04" |
| Lilith | 18 Gem 58' 18" |
| True Node | 00 Gem 47' 14" R |
| Planet | Aspect | Planet | Orb |
| Venus | Sextile | Neptune | 0.04 |
| Venus | Quincunx | Lilith | 1.82 |
| Mars | Opposition | Lilith | 2.59 |
| Jupiter | Square | True Node | 0.67 |
| Saturn | Square | Uranus | 1.52 |
| Uranus | Square | Saturn | 1.52 |
| Neptune | Sextile | Venus | 0.04 |
| Neptune | Square | Lilith | 1.78 |
| Lilith | Quincunx | Venus | 1.82 |
| Lilith | Opposition | Mars | 2.59 |
| Lilith | Square | Neptune | 1.78 |
| True Node | Square | Jupiter | 0.67 |
| January 5, 2022 | We |
![]()
![]()
![]()
![]()
![]()
![]() 01:44
01:44![]() VOC 01:44 - 24:00
VOC 01:44 - 24:00![]()
![]()
![]() 16:58
16:58
Re: ![]()
![]()
| January 6, 2022 | Th |
![]()
![]()
![]()
![]() VOC 00:00 - 01:16
VOC 00:00 - 01:16![]()
![]()
![]() 01:16
01:16![]()
![]() 03:59
03:59![]()
![]()
![]() 20:08
20:08
Re: ![]()
![]()
Janvier 2022
The Mansions of the Moon
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28
The Lunar Days
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 26 27 28 29 30
Jan 02 2022 8:09 Dim
Jan 03 2022 3:13 Lun
Jan 03 2022 12:35 Lun
Jan 05 2022 17:02 Mer
Jan 09 2022 1:47 Dim
Jan 10 2022 3:14 Lun
Jan 11 2022 4:27 Mar
Jan 11 2022 22:42 Mar
Jan 14 2022 12:42 Ven
Jan 16 2022 15:50 Dim
Jan 18 2022 10:54 Mar
Jan 18 2022 16:27 Mar
Jan 18 2022 19:48 Mar
Jan 19 2022 22:31 Mer
Jan 20 2022 3:38 Jeu
Jan 23 2022 11:27 Dim
Jan 23 2022 13:31 Dim
Jan 24 2022 13:52 Lun
Jan 26 2022 4:06 Mer
Jan 29 2022 3:56 AM
Jan 29 2022 5:19 AM
Jan 29 2022 9:47
Jan 30 2022 8:31
in 4.5 wks Feb 04 2022 5:14 Ven
in 4.6 wks Feb 04 2022 14:37 Ven
in 4.6 wks Feb 04 2022 20:04 Ven
in 4.8 wks
in 5.1 wks
in 5.6 wks Feb 11 2022 2:59 PM
in 5.7 wks
in 6 wks
in 6 wks Feb 14 2022 10:50 PM
in 6.2 wks
in 6.3 wks
in 6.5 wks
in 6.6 wks Feb 18 2022 5:42 PM
in 1.7 mnths
in 1.7 mnths
in 1.7 mnths
in 1.7 mnths
in 1.9 mnths Feb 28 2022 4:32 PM
in 1.9 mnths Mar 01 2022 4:54 AM
in 1.9 mnths Mar 02 2022 6:04 AM
in 1.9 mnths Mar 02 2022 17:31 Mer
in 2 mnths Mar 03 2022 9:42 Jeu
in 2 mnths Mar 03 2022 18:55 Jeu
| January 5th, 2022 | |
| The Sun is in Capricorn | |
| The Moon is in Aquarius The Crescent Moon is in Aquarius, Enters Pisces 00:17 | |
| Next Mercury Retrograde Period Jan 14 - Feb 3 | |
| Planet | Starts | Ends | Planet | Starts | Ends |
|---|---|---|---|---|---|
 | 08:41 | 09:24 |  | 17:13 | 18:30 |
 | 09:24 | 10:06 |  | 18:30 | 19:48 |
 | 10:06 | 10:49 |  | 19:48 | 21:05 |
 | 10:49 | 11:32 |  | 21:05 | 22:22 |
 | 11:32 | 12:14 |  | 22:22 | 23:40 |
 | 12:14 | 12:57 |  | 23:40 | 00:57 |
 | 12:57 | 13:40 |  | 00:57 | 02:14 |
 | 13:40 | 14:22 |  | 02:14 | 03:31 |
 | 14:22 | 15:05 |  | 03:31 | 04:49 |
 | 15:05 | 15:48 |  | 04:49 | 06:06 |
 | 15:48 | 16:30 |  | 06:06 | 07:23 |
 | 16:30 | 17:13 |  | 07:23 | 08:41 |
Paris
Wed, Jan 5, 2022
MERCREDI05JANVIERSemaine 1 - Jour 5 | EdouardCPremier croissant
| Sunrise 08:41 |
| Sunset 17:07 |
| Twilight ends 19:03 begins 06:46 |
10%
3 days old
4th Lunar Day  11:06
11:06
This Lunar Day is propicious for giving or receiving advice, putting together a serious venture, but also betrayal or resentment.
Waxing Moon
A good time for long-term partnership and for starting the implementation of far-reaching plans.
Moon in Aquarius  10:48
10:48
Suitable energy to make individual choices, to work in groups, to be with friends, to become aware of social issues, to change attitudes, share ideas, do the old thing in a new way, research, understand, study, teach, technology, Astrology, the Net, the unexpected, encounters with strangers or strange, eccentric people, UFO sighting. Purchase of books, encyclopaedias, computer programs, computers, technology, electrical appliances.
Void of Course Moon ![]()
![]()
![]() 01:44
01:44
Moon's Last Aspect: Sextile Mars.
| Rise | Set | |
| Mercury | 09:53 | 18:38 |
| Venus | 08:39 | 17:53 |
| Moon | 11:06 | 20:28 |
| Mars | 06:40 | 14:58 |
| Jupiter | 10:56 | 21:11 |
| Saturn | 10:12 | 19:26 |
| Rise | Culm. | Set | |
| Sun | 08:41 | 12:54 | 17:07 |
| Moon | 11:06 | 15:42 | 20:28 |
| Civil | Naut. | Astr. | |
| Twilight ends | 17:44 | 18:24 | 19:03 |
| Astr. | Naut. | Civil | |
| Twilight begins | 06:46 | 07:24 | 08:04 |
| The Planets | |||
| Rise | Culm. | Set | |
| Mercury | 09:51 | 14:13 | 18:34 |
| Venus | 08:41 | 13:18 | 17:54 |
| Mars | 06:39 | 10:48 | 14:57 |
| Jupiter | 10:56 | 16:03 | 21:10 |
| Saturn | 10:12 | 14:48 | 19:25 |
| Uranus | 13:09 | 20:22 | 03:35 |
| Neptune | 11:34 | 17:16 | 22:57 |
| Mercredi 5 Janvier 2022 15h32 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
**Conjunctions to Select Fixed Stars on January 5, 2021.**
Aspects to Sun 15°Cp19 -22°33′
conjunct 15°Cp36 VEGA Charismatic, magical, lucky
Aspects to North Node 00°Ge46 +20°18′
conjunct 00°Ge18 ALCYONE Mystical but judgmental.
 |
Sun enters the next Sabian symbol 05/01 at 10:14 h (chart) |
JANUARY 5
Today’s transits inspire our idealism and imagination. Venus forms a sextile to Neptune, encouraging us to find the best in people and our relationships or pleasures. We approach one another more gently and sensitively than usual with this transit. We are inclined to see the spiritual dimensions of our relationships and treat others with more care and concern. This influence encourages us to open up and embrace loving, non-possessive feelings. The Moon continues its transit of humanitarian Aquarius until 7:17 PM EST when it enters empathetic Pisces. Tonight, the Moon aligns with Jupiter in Pisces, magnifying and expanding our compassionate feelings. We’re reaching for our joy, and we are seeking to grow, develop, and improve, primarily through embracing our feelings, compassion, or downtime.
The void Moon period occurs until the Moon enters Pisces today at 7:17 PM EST.
- The Moon is in Aquarius until 7:16 PM, after which the Moon is in Pisces.
- The void Moon period continues until 7:16 PM (since yesterday at 7:44 PM).
- The Moon is waxing and in its New phase until 8:26 PM, after which the Moon is in its Waxing Crescent phase.
- The New Moon occurred on the 2nd in the sign of Capricorn.

Date & Time: Jan 5 2022 7:16 pm
Event: Moon enters Pisces
Description: The Moon in Pisces
A time to observe, feel, and process. We may pay special attention to our dreams, both of the night and day variety, and the subtle areas of our lives. Focusing on creativity, philanthropy, spirituality, and artistry can be beneficial. Build your intuition, slow down and rest.
Date & Time: Jan 5 2022 8:23 am
Event: Tr-Tr Mon SSq Chi
Description: Transiting Moon SemiSquare Transiting Chiron
Hurt feelings may be opportunities for healing. Now is the time for building bridges, not burning them.
Date & Time: Jan 5 2022 10:46 am
Event: Tr-Tr Mon Cpl Cer
Description: Transiting Moon ContraParallel Transiting Ceres
Date & Time: Jan 5 2022 11:30 am
Event: Tr-Tr Mon Pll Sat
Description: Transiting Moon Parallel Transiting Saturn
Date & Time: Jan 5 2022 11:50 am
Event: Tr-Tr Mon Pll Ven
Description: Transiting Moon Parallel Transiting Venus
Date & Time: Jan 5 2022 2:21 pm
Event: Tr-Tr Mon Sxt Ves
Description: Transiting Moon Sextile Transiting Vesta
We can derive a nice feeling of satisfaction and fulfillment from tending to our responsibilities, work, or other commitment. We are more able to make sacrifices or put our emotions aside in order to get something important done. Alternatively, we can bring more sensitivity to our practical affairs. This can be a good time to commit fully to something – especially health and healing matters. It’s also a strong time for taking care of domestic matters.
Date & Time: Jan 5 2022 4:17 pm
Event: Tr-Tr Mon Sqr Cer
Description: Transiting Moon Square Transiting Ceres
There can be conflicts with loved ones, or we could be feeling unsupported. People could seem insensitive or uncaring, or our own needs seem to be at odds with the needs of family and friends.
Date & Time: Jan 5 2022 7:16 pm
Event: Tr-Tr Mon Cnj Pis
Description: Transiting Moon Entering Pisces
A time to observe, feel, and process. We may pay special attention to our dreams, both of the night and day variety, and the subtle areas of our lives. Focusing on creativity, philanthropy, spirituality, and artistry can be beneficial. Build your intuition, slow down and rest.
Date & Time: Jan 5 2022 8:26 pm
Event: Tr-Tr Mon SSq Sun
Description: Transiting Moon SemiSquare Transiting Sun
What we feel and what we think we should do can be at odds with one another temporarily. There can be discontent or indecisiveness. We could be feeling out of sorts. Tensions will pass.
Date & Time: Jan 5 2022 8:31 pm
Event: Tr-Tr Mon Sqr Nod
Description: Transiting Moon Square Transiting North Node
This is the trigger for out with the old and in with the new. We could feel at odds with others on an emotional level. Public relations are not as favorable now. Plans could need reworking. We could feel temporarily out of the loop.
Date & Time: Jan 5 2022 9:56 pm
Event: Tr-Tr Mon Cnj Jup
Description: Transiting Moon Conjunction Transiting Jupiter
We want to do good and to honor our inner code. We are generous with our energy, time, and money. Our feelings expand or are magnified, for better or for worse. It’s generally a good time to teach, learn, write, express ourselves, reach out, travel, publish, and promote. We may go over the top.
Date & Time: Jan 5 2022 9:26 pm
Event: Tr-Tr Sun Sqq Nod
Description: Transiting Sun SesquiSquare Transiting North Node
This can sometimes correlate with a need to let go of a project, relationship, attitude, or conditions that have now become troublesome or counterproductive.
Date & Time: Jan 5 2022 1:24 pm
Event: Tr-Tr Mer Cpl Nod
Description: Transiting Mercury ContraParallel Transiting North Node
Date & Time: Jan 5 2022 11:02 am
Event: Tr-Tr Ven Sxt Nep
Description: Transiting Venus Sextile Transiting Neptune
We are more sensitive to one another’s feelings and needs, and more aware of our higher or non-material needs at this time.
CAPRICORN STRONG
Disciplined, responsible, reliable, industrious, conscientious, practical, achieving. Can be pessimistic, overly conventional, rigid, materialistic, callous.
AQUARIUS STRONG
Humanitarian, innovative, group conscious, progressive, serving others. Can be rebellious, eccentric, aloof, emotionally superficial, overly extroverted.
Today’s Elemental Balance
FIRE WEAK
We are not very goal-oriented right now, or motivation to pursue our goals may be waning/lacking. Changes feel overwhelming. Enthusiasm may be low, we argue less, and we think more than we take action.
EARTH STRONG
Earth signs are Taurus, Virgo, and Capricorn. We are especially in touch with the physical world. We consider what we’ve learned and experienced in the past in order to make the most of the present. We can be cautious, practical, and possibly unimaginative. We are deliberate and can pace ourselves well. We need hands-on experience and are not impressed with theory as much as we are with results. Routines are tolerable and comforting.
WATER WEAK
There may be some insensitivity or lack of empathy now. We may be out of touch with what we’re feeling or with our emotional needs. We may be tougher than usual now, and more inclined to follow mental or practical considerations than we are to listen to our intuition.
Today’s Modal Balance
The modes are balanced today.
Today’s Lunar Phase
LUNAR PHASE: NEW MOON
Moon 0 to 45 degrees ahead of the Sun.
This is an energetic, impulsive, and subjective period of time. We are looking for new projects and opportunities.
THE MOON IN AQUARIUS
Our reactions are more intellectual than emotional, at least on the surface of things, and interactions are more impersonal than personal. Social gatherings, dealing with group ideals and goals for the future, brainstorming, new ideas, and progressive changes are in focus.
26TH DEGREE OF AQUARIUS
Part of Body: Right fibula
Sabian Symbol: A hydrometer.
ASPECTS OF THE MOON
CONJUNCTION JUPITER Orb 5°46′ Applying
We want to honor our inner code. We are generous with our energy, time, and money. Our feelings expand or are magnified, for better or for worse. It’s generally a good time to teach, learn, write, express ourselves, reach out, travel, publish, and promote. We may go over the top, however.
THE SUN
THE SUN IN CAPRICORN
You are responsible and respectful, with a strong need to be an authority figure. You command respect and may tend to superiority and bossiness.
16TH DEGREE OF CAPRICORN
Part of Body: Condyle of right tibia
Sabian Symbol: Boys and girls in gymnasium suits.
ASPECTS OF THE SUN
CONJUNCTION VENUS Orb 5°24′ Applying
We take pride in our ability to relate well with others or to smooth over differences now. Graciousness, diplomacy, charm, and some superficiality are themes. This is a good period for social affairs, pleasure, amusement, and romance, all things considered.
TRINE URANUS Orb 4°26′ Separating
We are looking forward, happy and confident about making changes and improvements. We are willing to experiment and explore.
MERCURY
MERCURY IN AQUARIUS
You are brimming with original and unique ideas. You enjoy exchanging ideas with other people, particularly friends and groups of people. You are an inventive thinker, and may rebel against old and traditional ideas.
5TH DEGREE OF AQUARIUS
Part of Body: Nerve of right fibula
Sabian Symbol: A council of ancestors.
ASPECTS OF MERCURY
CONJUNCTION SATURN Orb 8°02′ Applying
You have a logical mind. As a child you were shy and unable to express your opinions. As an adult you will become a voice of authority.
VENUS
VENUS IN CAPRICORN
There can be some reticence about forming new connections, but when we do form them, they are longterm. Getting serious about our finances and boosting our relations with business partners and/or co-workers can be in focus. We value reliability. We take expressing our feelings seriously, and we take our time to warm up to others. Our tastes are traditional.
21ST DEGREE OF CAPRICORN
Part of Body: Tendons of left knee
Sabian Symbol: A relay race.
ASPECTS OF VENUS
SEXTILE NEPTUNE Orb 0°01′ Separating
You and a romantic and gentle partner. You like to share creative pursuits in your relationships.
CONJUNCTION PLUTO Orb 5°21′ Separating
You want intensity and drama in your personal relationships. You are consumed with love and passion and expect your partner to feel the same way. You are disappointed when others fail to soar to great emotional heights and plummet the emotional depths by your side. On a more positive note you are fiercely loyal and committed with an ability to fight any injustice.
MARS
MARS IN SAGITTARIUS
You are an inspirational leader, and have a strong desire for exploration and adventure. You may be one-pointed in your desires.
17TH DEGREE OF SAGITTARIUS
Part of Body: Sciatic nerve
Sabian Symbol: An easter sunrise service.
ASPECTS OF MARS
SQUARE NEPTUNE Orb 4°18′ Applying
This is a time for resting, escaping, or taking a break as we reconsider our needs. We might temporarily lose focus or direction.
JUPITER
JUPITER IN PISCES
You are on an intuitive search for the truth. You are a champion of the underdog. You could be a spiritual or religious teacher.
2ND DEGREE OF PISCES
Part of Body: Left calcaneum
Sabian Symbol: A squirrel hiding from hunters.
SATURN
SATURN IN AQUARIUS
You have an ability to work well in groups. Your work is often original and innovative.
13TH DEGREE OF AQUARIUS
Part of Body: Right tibial artery
Sabian Symbol: A barometer.
ASPECTS OF SATURN
SQUARE URANUS Orb 1°31′ Separating
You work to overcome a feeling of inadequacy about your abilities. You have problems with authority figures and need to learn to compromise. When you have done this you will succeed, particularly in large organisations.
URANUS
URANUS IN TAURUS
(1934 – 1942) We approach money and personal possessions in new ways and learn how to free ourselves from certain material constraints. Innovative ways to make ourselves comfortable emerge. We’re less inhibited about expressing sensuality, self-love, body love, and pampering. We’re challenging what we previously valued. There can be abrupt changes with money, valuables, possessions, and income that lead to a reshuffling of priorities or values. Income might come from non-traditional sources or ventures. We’re bringing progressive ideas to the world of business. New ways of doing business, as well as making, viewing, and handling money are likely. Income and the energy we put into making money can be variable. (May 15, 2018, to November 6, 2018, then March 6, 2019, to July 7, 2025, and then November 7, 2025, to April 25, 2026).
11TH DEGREE OF TAURUS
Part of Body: Cervical and brachial plexi
Sabian Symbol: A woman sprinkling flowers.
NEPTUNE
NEPTUNE IN PISCES
A long-term influence in which fantasy, imagination, compassion, and spirituality are in stronger focus. (April 4, 2011, to August 4, 2011, then February 3, 2012, to March 30, 2025, then October 22, 2025, to January 26, 2026)
21ST DEGREE OF PISCES
Part of Body: Left fibula muscle
Sabian Symbol: A little white lamb, a child, and a servant.
PLUTO
PLUTO IN CAPRICORN
Tests of our boundaries; breaking down and rebuilding structures and rules. (From January 25, 2008, to June 14, 2008, then November 26, 2008, to March 23, 2023, then June 11, 2023, to January 20, 2024, then September 1, 2024, to November 19, 2024).
27TH DEGREE OF CAPRICORN
Part of Body: Deep nerves
Sabian Symbol: A mountain pilgrimage.
CHIRON IN ARIES
Your sense of being has been violated in some way and you may fear asserting yourself. You may also over-compensate by attempting to be the first at everything. Physically you may suffer head wounds. You may become a pioneer in a way that will be of service to humanity.
9TH DEGREE OF ARIES
Part of Body: Lens of eye
Sabian Symbol: A crystal gazer.
VESTA IN SAGITTARIUS
You have the ability to work relentlessly for a cause in which you believe. You need to make sure you do not exclude other people’s points of view. You have visions and enjoy the adventure of discovery in your work.
28TH DEGREE OF SAGITTARIUS
Part of Body: Right leg muscles
Sabian Symbol: An old bridge over a beautiful stream.
PALLAS IN PISCES
You have a meditative mind, the ability to merge in your mind with the universe. You desire to heal the world through words, and may be involved in some form of artistic expression.
18TH DEGREE OF PISCES
Part of Body: Extensor muscles of the right toes
Sabian Symbol: In a huge tent a famous revivalist conducts his meeting.
JUNO IN CAPRICORN
You want a partner who you can respect, and who respects you. You seek long-term commitment, and may marry later in life.
20TH DEGREE OF CAPRICORN
Part of Body: Tendons of right knee
Sabian Symbol: A hidden choir singing.
CERES IN TAURUS
You feel cared for when loved ones give you a sense of stability and security. You like to be touched and stroked, and nurtured in practical ways. You also like to show others you care through practical means.
29TH DEGREE OF TAURUS
Part of Body: Deltoid muscle and main neck muscles
Sabian Symbol: Two cobblers working at a table.
THE BLACK MOON IN GEMINI
19TH DEGREE OF GEMINI
Part of Body: Laryngeal muscles
Sabian Symbol: A large archaic volume.
ERIS IN ARIES
24TH DEGREE OF ARIES
Part of Body: Zygomatic muscle
Sabian Symbol: An open window and a net curtain blowing into a cornucopia.
THE NORTH NODE
THE NORTH NODE IN GEMINI
This is a quest to learn to see life from other people’s point of view and to become adept in the art of communication. You have a tendency to be restless and scattered. You need to develop focus and the ability to communicate your ideas in a way that helps the many people in your life.
1ST DEGREE OF GEMINI
Part of Body: Trachea
Sabian Symbol: A glass-bottomed boat drifts over under-sea wonders.
THE SOUTH NODE
THE SOUTH NODE IN SAGITTARIUS
This is a quest to learn to see life from other people’s point of view and to become adept in the art of communication. You have a tendency to be restless and scattered. You need to develop focus and the ability to communicate your ideas in a way that helps the many people in your life.
1ST DEGREE OF SAGITTARIUS
Part of Body: Pelvic bone
Sabian Symbol: A grand army of the republic campfire.
This Week’s Aspects
![]()
![]()
![]() Venus sextile Neptune. We are more imaginative and attuned to the world of beauty and romance today. Gentleness with others is the best way to harness this energy. A “magical” time on a romantic and social level is possible now.
Venus sextile Neptune. We are more imaginative and attuned to the world of beauty and romance today. Gentleness with others is the best way to harness this energy. A “magical” time on a romantic and social level is possible now.
![]()
![]()
![]() Mercury semi-square Neptune. We are not thinking clearly, preferring to daydream. Technical facts can be glossed over at this time. It’s not a good time to make a presentation, to ask for what you want, or to formalize agreements.
Mercury semi-square Neptune. We are not thinking clearly, preferring to daydream. Technical facts can be glossed over at this time. It’s not a good time to make a presentation, to ask for what you want, or to formalize agreements.
![]()
![]()
![]() Sun semi-square Jupiter. We may feel vague restlessness and discontent with life as it is. We want more, but perhaps too much. Overestimation, wastefulness, and exaggeration. Elevated moods, but unstable ones, as they may not be based on reality.
Sun semi-square Jupiter. We may feel vague restlessness and discontent with life as it is. We want more, but perhaps too much. Overestimation, wastefulness, and exaggeration. Elevated moods, but unstable ones, as they may not be based on reality.
![]()
![]()
![]() Venus conjunct Sun. We are motivated by the desire to strike a balance in our relationships and in our environment. Smooth negotiations with others. Graciousness, diplomacy, charm, and some superficiality. This is a good period for social affairs, pleasure, amusement, and romance, all things considered.
Venus conjunct Sun. We are motivated by the desire to strike a balance in our relationships and in our environment. Smooth negotiations with others. Graciousness, diplomacy, charm, and some superficiality. This is a good period for social affairs, pleasure, amusement, and romance, all things considered.
Sun in Capricorn You see what’s wrong and you know what to do now: the time has come to make it real, and it’s a lot bigger than any one person. This means cooperation and organization, authority and discipline, responsibility – and all these things are what it takes to get you where you know you must go. But take care! If your vision is only for yourself, what you create cannot last: it must be for some greater good. Ambition, practicality, and achievement are admirable, but they are means to an end – not ends in themselves.
Mercury in Capricorn Figuring out how to organize projects and people is apt to become a topic of special interest – and a challenge – at a time like this. Opinions are not enough for you now: they must be backed by authority or evidence, and above all they must have practical worth and application. A period of intense study and thought.
Mercury in Aquarius The tried and true doesn’t cut it anymore: it’s the new, the unique, and the original that catches and holds your interest now. Idealism, rationality, and critical thinking become more and more a part of your mental patterns and the way you communicate. You are inventive, original, high tech.
Venus in Capricorn Marry for money and then learn to love ’em: that’s what your heart tells you now, as a new cycle gets underway in your life. Does that sound cynical? Maybe so – but it does reflect the yearning you feel for security and stability now, and it suggests the kind of compromises you may be willing to make to accomplish these objectives.
Mars in Sagittarius Ideology is a keynote of the new phase you have begun – the power of ideas and the power they wield over people. Religious, cultural, or philosophical controversies and crusades have a way of stirring your blood. Travel and adventure are compelling temptations as you dare to test your mettle and stretch your horizons.
Jupiter in Pisces Mysticism, sensitivity to the needs of others, and an awareness of karma (the link between the present and the past): these are your paths to spiritual growth now. Addressing these issues brings you gain and satisfaction at many levels. A tolerance for differences, an understanding of human frailties and shortcomings.
Saturn in Aquarius Ideals are in for a period of testing, as a new phase begins in your life. What are principles worth, unless they are put into practice? That’s an important question for you now. This could mean you may be disappointed by causes you believe in – but if you abandon your principles, you lose.
Uranus in Taurus Practicality and ingenuity combine as powerful drives in your life now that you have begun a new cycle. Taking innovative concepts and making them real and practical is your strong suit; getting stuck in the mud is to be avoided at all costs. Financial savvy, inventiveness, worldly genius.
Neptune in Pisces Things spiritual and psychic take the driver’s seat of your imagination as you begin a new cycle. The fine arts and all manner of fantasies assume a larger-than-life importance. Surrendering to fate, karma, and other powers greater than merely mortal may be seen as the ultimate liberation. If all is Maya (illusion), what is the reality that gives rise to the world of appearances?
Pluto in Capricorn As this new phase dawns for you, the pursuit of social/political power and status has a way of seeming necessary and inevitable – and let nothing stand in its way. It starts out small but has bigger consequences than you’d think. Ambition, responsibility, a place for everyone, and everyone in their place…
This week: The Sun is in Capricorn; Mercury is in Capricorn to the 2nd, when Mercury moves into Aquarius; Venus is in Capricorn; Mars is in Sagittarius.
The Sun is in Capricorn from December 21, 2021-January 19, 2022. With the Sun in Capricorn, we are motivated by feelings of responsibility, ambition, and respect for law and order. Capricorn derives much satisfaction in completion and accomplishment. Standards, structures, and an appreciation for order are Capricorn themes.
Capricorn wants tangible results, knows what is feasible and what is not, and is most comfortable working within an established framework and known boundaries or limits. Working towards a long-term goal is most satisfying with this influence.
Mercury is in Capricorn from December 13, 2021-January 2nd, 2022. When Mercury is in Capricorn, our thinking is methodical and our focus is sober and practical. It is easier to concentrate on the task at hand under this influence. Our conversations may be quite realistic or focused on business/practical matters. Our thought patterns and communication styles become more logical, orderly, and organized.
We are more able to sort out what is relevant and essential. Realism enters the picture, our speech is no-nonsense, and precision becomes important to us. Our survival instincts are strong–we’re interested in what will benefit us in the long term.
Mercury is in Aquarius from January 2-25, 2022, and then later, from February 14-March 9, 2022. When Mercury is in Aquarius, we are hungry for knowledge and open to new and original ideas. We begin to think outside of the box, and our thinking becomes more progressive and objective. Our communication and thought patterns are spontaneous, somewhat fragmented, reasonable, objective, and liberal. This is a time when inventive thinking is at a peak.
Venus is in Capricorn from November 5, 2021-March 6, 2022. [This is longer than usual due to the Venus retrograde, occurring entirely in the sign of Capricorn, from December 19, 2021-January 29, 2022.] Getting serious about our finances and boosting our relations with business partners and/or co-workers will be easier than usual under this influence. Our talents for creating artistic structure and form are enhanced, and our ability to form connections with people who support our ambitions increases.
We value enduring connections, reliability, and faithfulness. We take expressing our feelings seriously, and we take our time to warm up to others. We generally keep our “cool” in relationships. We’re steadfast and seek genuine, perhaps old-fashioned connections. It’s not a time for PDA, and we can be somewhat guarded and reserved with expressing our feelings or revealing our relationships.
The underdog who works hard can have their time in the sun now. We value slow and steady effort and long-term prospects. Reliability in others is most attractive.
This cycle favors getting along well with higher-ups, conservative business goals and ventures, long-term investments, and matters related to pension.
Mars is in Sagittarius from December 13, 2021, to January 24, 2022. With a Sagittarius Mars, our actions are motivated by our ideals. We are bothered by routine, quickly becoming restless if we feel confined. We have a love of adventure, and of conquest–this is the position of wanderlust. We start projects or challenges with gusto, although we may tend to abandon them rather quickly. This may be because we set our sights too high!
![]()

Jupiter is in Pisces from December 28th, 2021, to May 10, 2022. Jupiter returned to Pisces on December 28th, 2021. There will be another part to this 3-part act in 2022! Read more about the Jupiter in Pisces transit.
![]()

Saturn transits Aquarius from March 21 to July 1, 2020, and from December 17th, 2020, until March 7, 2023. Read about Saturn’s transit of Aquarius.

![]()
Uranus is in Taurus.
Uranus transits Taurus from May 15, 2018, to November 6, 2018, and then from March 6th, 2019, until 2025/6. We approach money and personal possessions in new ways and learn to free ourselves from certain material constraints. Innovative ways to make ourselves comfortable emerge. We’re less inhibited about expressing sensuality, self-love, body love, and pampering. Above all, we’re challenging what we previously valued during this cycle.
There can be abrupt changes with money, valuables, possessions, and income that lead to a reshuffling of priorities or values. Income might come from non-traditional sources or ventures. In general, we’re bringing progressive ideas to the world of business. New ways of doing business, as well as making, viewing, and handling money, are likely. Our income and the energy we put into making money can be variable.
Romance & Relationships
Venus is in Capricorn from November 5, 2021, to March 6, 2022. Venus in Capricorn is a cycle in which security and stability in our relationships are paramount. We are more cautious with our emotions and somewhat skeptical of both our own feelings and others’ expressions of affection. Longevity is important to us under this influence.
There is a formality to Capricorn that can make personal expressions seem awkward and forced. However, this cycle offers us the opportunity to consider some of the practical issues of our relations and to work on making our partnerships work in the real world. A dose of realism in our personal lives can help us to feel more grounded and secure in the long run.
This week: Venus is retrograde. Venus-ruled endeavors, such as money, beauty, art, romance, and pleasure, become a little heavier, more prone to reassessment, review, and perhaps delay or a period of limbo. This Venus retrograde cycle lasts until January 29th, and is a period in which we reevaluate or review our love life as well as the manner in which we receive, reveal, and express our affections. Thoughts turn to the past so that we can lay to rest unhealthy habits in these areas of life.
With Venus retrograde in Capricorn, we may question whether our goals mesh in our relationships. Read more about Venus retrograde in Capricorn from December 19, 2021, to January 29, 2022.
On Tuesday, we’re getting serious about our feelings or relationships. Responsibilities are in question. On Wednesday, however, we’re shifting gears as we recognize the important non-material elements of our bonds with others. It’s a romantic time when nostalgia is a draw.
On Saturday, January 8th, we see the past in a new light, particularly related to our relationships and pleasures. We can be symbolically in the dark when it comes to our feelings or relationships as a new cycle begins. This is a time for redefining what we want and need, particularly from intimate relationships but also related to our material affairs and goals. We are infinitely more invested in our ability to harmonize, love, connect, and relate.
Venus, the Goddess of Love: Highlights in the Coming Week:
![]() Venus in Capricorn Marry for money and then learn to love ’em: that’s what your heart tells you now, as a new cycle gets underway in your life. Does that sound cynical? Maybe so – but it does reflect the yearning you feel for security and stability now, and it suggests the kind of compromises you may be willing to make to accomplish these objectives.
Venus in Capricorn Marry for money and then learn to love ’em: that’s what your heart tells you now, as a new cycle gets underway in your life. Does that sound cynical? Maybe so – but it does reflect the yearning you feel for security and stability now, and it suggests the kind of compromises you may be willing to make to accomplish these objectives.
Venus conjunct Mercury December 28-29. Favors playfulness in love and enhanced communication between partners.
Venus parallel Saturn January 4. We may express our affection sparingly, but also responsibly and in practical ways. Relationships begun under this influence often feel burdensome over time, but they may last longer than most.
Venus sextile Neptune January 4-5. Unusual attractions that add imagination, inspiration, and emotional “color” to our lives may occur now. We are attuned to the subtleties of human interaction, and we are more inclined to naturally cooperate with others. An imaginative, romantic time.
Venus conjunct the Sun January 8. Good relating is the focus, although some may find this energy superficial in that surface charm, diplomacy, and harmony is the goal. The pursuit of pleasure is emphasized.
Venus semi-square Jupiter January 10. We experience a strong desire for more pleasure in our lives, which can be excessive. Take care not to overstate your feelings or to set up unrealistic expectations in your partnerships.
The purple marker above shows where we are in the current moon phase cycle.
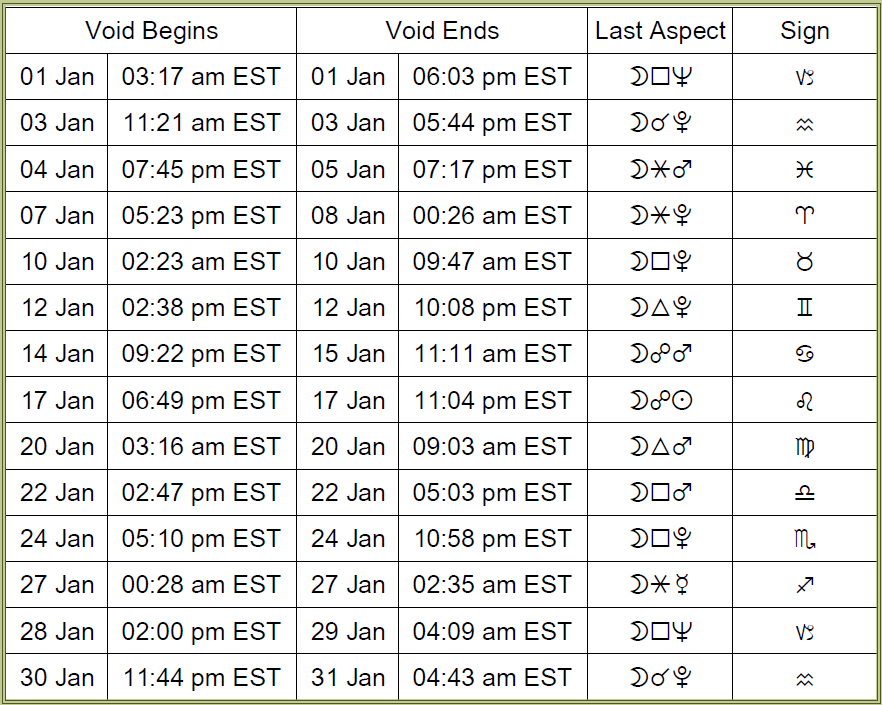
Void-of-course Moon on Monday, January 3rd, from 11:21 AM EST, with the Moon’s last aspect before changing signs (a conjunction to Pluto), until the Moon enters Aquarius at 5:44 PM EST.
VOC Moon on Tuesday, January 4th, from 7:45 PM EST, with the Moon’s last aspect before changing signs (a sextile to Mars), until the Moon enters Pisces the next day, Wednesday, January 5th, at 7:17 PM EST.
VOC Moon on Friday, January 7th, from 5:23 PM EST, with the Moon’s last aspect before changing signs (a sextile to Pluto), until the Moon enters Aries the next day, Saturday, January 8th, 12:26 AM EST.
January 2022:
Timing with the Moon – Better Periods this Week for Electing New Initiatives
It’s important to note that there are more factors to consider in addition to the windows listed below. The following windows are starting points. For example, you generally don’t want to elect a new venture when a very challenging aspect is applying, and it’s preferable to elect a time when a particularly positive aspect hasn’t yet perfected (is applying rather than separating).
The Moon is waning this week until the New Moon on the 2nd, which is generally considered unfavorable for new beginnings, with exceptions. (See Best Time to Start a Business for more details). The Moon is waxing this week after the New Moon on the 2nd, which is generally considered favorable for new beginnings, also with exceptions.
According to principles of timing with the Moon, a window of opportunity occurs this week at Cazimi Moon on Sunday, January 2nd, from approximately 1:05 PM to 2:00 PM EST, while the Moon is in Capricorn. Another window occurs on Friday, January 7th, from approximately 4:00 AM to 5:20 PM EST, while the Moon is in Pisces. Note that Venus is retrograde during these windows.
See also the following articles and tables: Timing with the Moon and Opportunity Periods with the Void Moon for times and techniques.
Planetary Stations & Sign Ingresses:
Nov 5, 2021 6:44 AM Venus enters Capricorn
Nov 5, 2021 6:35 PM Mercury enters Scorpio
Nov 8, 2021 4:24 AM Pallas Direct
Nov 14, 2021 3:23 PM Juno enters Capricorn
Nov 16, 2021 9:04 AM Vesta enters Sagittarius
Nov 21, 2021 9:34 PM Sun enters Sagittarius
Nov 24, 2021 10:36 AM Mercury enters Sagittarius
Dec 1, 2021 8:22 AM Neptune Direct
Dec 13, 2021 4:53 AM Mars enters Sagittarius
Dec 13, 2021 12:52 PM Mercury enters Capricorn
Dec 19, 2021 5:36 AM Venus Retrograde
Dec 19, 2021 11:32 AM Chiron Direct
Dec 21, 2021 5:52 AM Ceres Retrograde enters Taurus
Dec 21, 2021 10:59 AM Sun enters Capricorn
Dec 28, 2021 11:09 PM Jupiter enters Pisces
Jan 2, 2022 2:09 AM Mercury enters Aquarius
Jan 11, 2022 12:16 AM Vesta enters Capricorn
Jan 14, 2022 6:41 AM Mercury Retrograde
Jan 14, 2022 4:21 PM Ceres Direct
Jan 18, 2022 10:25 AM Uranus Direct
Jan 18, 2022 1:50 PM True North Node enters Taurus
Jan 19, 2022 9:39 PM Sun enters Aquarius
Jan 24, 2022 7:53 AM Mars enters Capricorn
Jan 25, 2022 10:05 PM Mercury Rx enters Capricorn
Jan 29, 2022 3:46 AM Venus Direct
Jan 2, 2022, 1:33 PM, Sun Conjunct Moon (New Moon)
Jan 9, 2022, 1:11 PM, Sun Square Moon (First Quarter Moon)
Jan 17, 2022, 6:48 PM, Sun Opposition Moon (Full Moon)
Jan 25, 2022, 8:41 AM, Sun Square Moon (Last Quarter Moon)
Feb 1, 2022, 12:46 AM, Sun Conjunct Moon (New Moon)

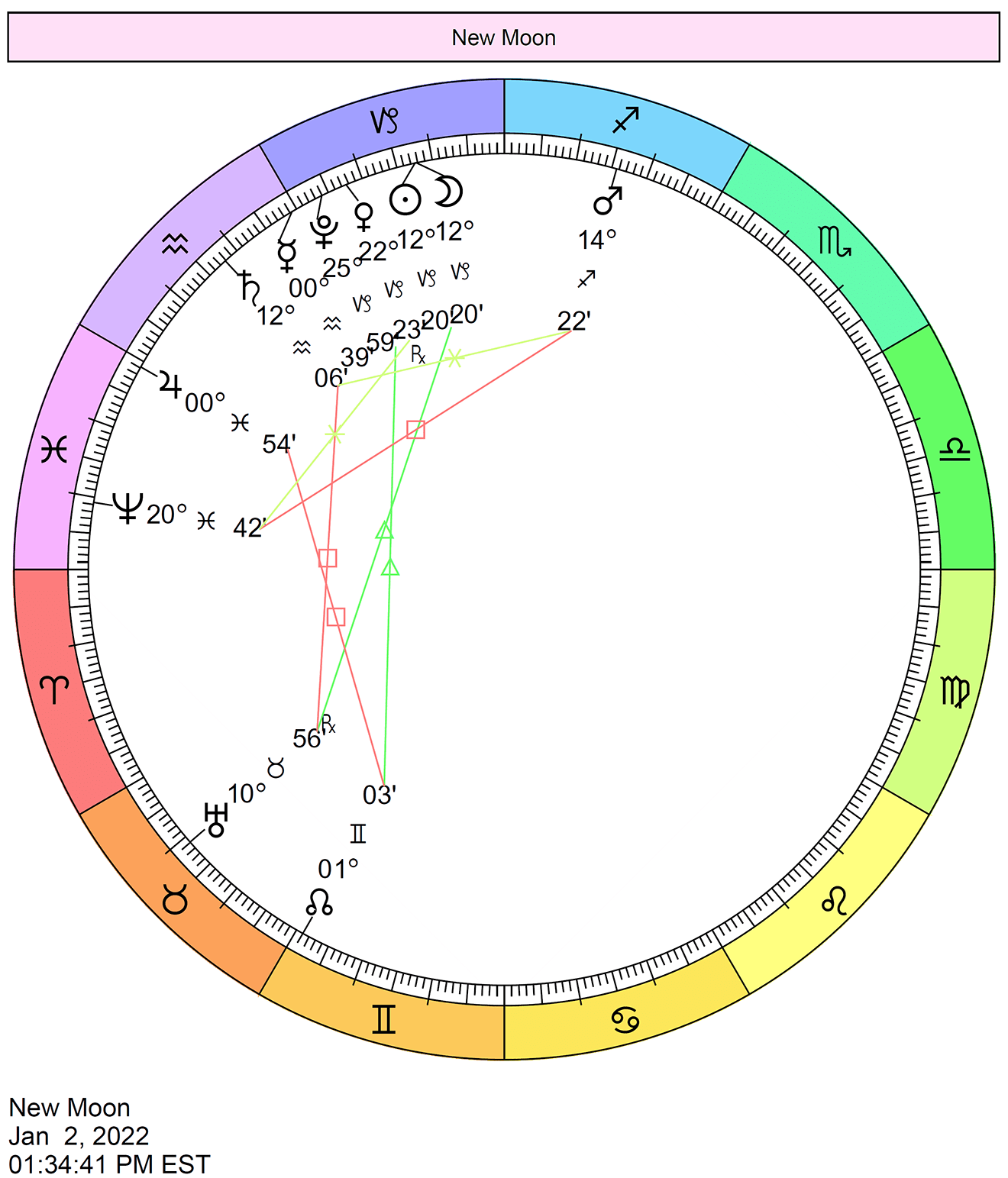
The NEW MOON occurs on Sunday, January 2, 2022, at 19:33.
The New Moon in Capricorn marks the beginning of a new cycle. The Sagittarius New Moon cycle ends and the Capricorn New Moon cycle begins.
The New Moon is exact on the afternoon of January 2nd when the Sun and Moon align in the sign of Capricorn. This lunation symbolizes a new beginning in the cardinal earth sign of Capricorn. It’s a time during which we can focus on some of the constructive traits of the sign of the Goat–tenacious, resourceful, disciplined, wise, ambitious, prudent, constant–and consider how to positively incorporate these qualities into our lives. Now, we can focus on practical and attainable long-term goals, work on developing maturity and common sense, prepare for the future, make commitments and recognize responsibilities, and nurture our dreams in a realistic manner.
With this potent Capricorn energy, we have the chance to create some serious order in our lives. It’s time to make some solid plans and to set the stage for reaping the rewards from our new beginnings, as little or big as they may be, in approximately two weeks’ time after the Full Moon occurs. One of Capricorn’s “lessons” is acceptance of the concept that reasonable boundaries and rules actually do offer us freedom–freedom from worry and chaos, for example. Setting goals for ourselves allows us to focus on what really matters.
This lunation harmonizes beautifully with Uranus, pointing to strong intuition and a good feeling of progress, as well as a healthy relationship with change.
This phase of the Moon occurs at 12 degrees and 20 minutes of Capricorn, affecting people with personal planets and points at approximately 8 to 16 degrees of the Cardinal signs (Aries, Cancer, Libra, and Capricorn) most significantly.
![]()
![]()
The Moon is traveling through Capricorn today. Make a list of goals. Work overtime. Climb higher. Don't sulk.
We become aware of the need for structure and planning ahead with a Capricorn Moon. We are instinctively aware of the limitations of time and motivated by a desire for success. Achievement and manifestation are more important to us now. We are resourceful and don't want to waste time, energy, or resources. This can be a somewhat sober influence, but it can also be a productive time when we look reality in the eye.
The Moon in Capricorn generally favors the following activities: Long-term activities that yield slow but steady results, practical undertakings, career issues, making a business plan, practical investments.
JANUARY 6
Today’s Pisces Moon is gentle, creative, and compassionate. It’s a good time for winding down or taking a break from the usual pace. Two minor challenging aspects suggest some misjudgment or restlessness today. We want to grow, improve, and expand, and it may be necessary to push some boundaries now, but it’s best to do so gently. We should watch that we aren’t idealizing what we don’t have with people, situations, and things. There can be cloudy thinking or a tendency to daydream. We may not express ourselves clearly, or others are not reading our messages well–likely, it’s a combination of the two!
JANUARY 7
The Moon spends the day in Pisces, encouraging us to connect with our imaginative, compassionate side. We’re tolerant and mellow, and we’re easily touched. We quickly tune into the vibrations around us, and we’re psychically open. We’re inclined to dream, inspire, and seek inspiration. While we could deal with motivation levels dipping, it’s likely temporary, and it can stimulate us to get more in touch with our desires.
The void Moon occurs from 5:23 PM EST, with the Moon’s last aspect before changing signs (a sextile to Pluto), until the Moon enters Aries the next day, Saturday, January 8th, 12:26 AM EST.
JANUARY 8
The void Moon period continues until the Moon enters Aries today at 12:26 AM EST.
 |
Aujourd'hui3° / 6° Risque de pluie : 84% - Humi. : 66% Vent :Ouest-Nord-Ouest - 17 km/h Lever : 08:44 - Coucher : 17:09 Possibles averses de pluie |
| HOROSCOPE DU MERCREDI 5 JANVIER 2022 | |||
| BELIER Ne vous laissez pas influencer par vos amis pour ce qui concerne votre avenir professionnel, mais sachez vous remettre en question si des doutes subsistent... | BALANCE Votre cr�ativit� est au top et vous trouvez l'inspiration dans votre propre famille, ou en prenant plaisir � travailler � la maison. Bon rapport avec les enfants. | ||
| TAUREAU Si vos pens�es sont n�gatives, c'est sans doute parce que l'avenir vous fait peur, notamment dans le domaine socioprofessionnel. Remettez-vous au pr�sent � chaque seconde... | SCORPION M�me si les soucis familiaux vous prennent la t�te, les contacts et les rencontres professionnelles demeurent vos priorit�s. Faites la part des choses... | ||
| GEMEAUX Quand les r�ves d'horizons lointains rejoignent la r�alit� p�cuniaire, il y a parfois des lendemains qui d�chantent. A vous d'opter pour le juste milieu. | SAGITTAIRE En ce moment, vous ne tenez pas en place et la confiance semble revenir. Il faut dire que l'on vous appr�cie de plus en plus, que l'on tient compte de vos go�ts et de vos couleurs! | ||
| CANCER Si vous ne pensez qu'� l'argent qui doit rentrer, vous aurez du mal � appr�cier celui que vous allez sortir... lorsque vous inviterez votre partenaire ! L�chez prise... | CAPRICORNE Non seulement vous pouvez vous mettre en avant, mais vous avez la possibilit� de faire valoir votre savoir faire ou d'am�liorer vos comp�tences. Osez vous imposer! | ||
| LION Votre partenaire ou vos collaborateurs vous font sans doute remarquer que vous accordez plus de place � votre travail. Avant de devenir trop accro, ouvrez votre coeur! | VERSEAU Aujourd'hui vous fuyez les mondanit�s et la foule. Vous avez besoin de r�fl�chir sur vous-m�me, de vous recentrer. Ce n'est pas une raison pour bouder! | ||
| VIERGE Aujourd'hui, vous privil�giez l'amour, les plaisirs et les loisirs. Mais le travail et les obligations quotidiennes vous rappellent � l'ordre. Patience... | POISSONS Bien que vos projets se mettent en place et que vos amis vous sollicitent, vous �prouvez le besoin de prendre du recul, de m�diter en silence ou de vous reposer... | ||
Love & Money Calendar™
THE BEST ASTROLOGICAL DAYS THIS MONTH AND
THE WORST ASTROLOGICAL DAYS THIS MONTH
FOR SIXTEEN IMPORTANT ACTIVITIES FOR
January, 2022
| The dates below are the best (and worst) astrological days in January 2022 for the 17 important activities described (to read about the criteria we use in selecting these dates click here). January is another below average month when it comes to good and bad astrological days for natalizations. As was the case last month, the days we list as the "best" days of the month are not really "best", but are actually "barely good enough" – and unfortunately, none are good enough for any crucial natalizations. As always, please make certain you are not having bad transits when you make crucial natalizations. Our MagicalOracle Mobile Web App is the easiest way to know what kind of transits you have on any day and you can check it anywhere in the world at any time by using your iPhone or Android, notebook or PC. As for the best and worst days this month for money, they are: Best: January 1, 12 Worst: January 17, 26, 27 And the best and worst days this month for love and sex are: Best: January 1, 6, 28, 30 Worst: January 17, 18, 19 Please also always remember that in addition to selecting good astrological days for crucial natalizations, you need to have good transits at the same time in order to create a natalization that will work very well for you. If you are a member of the Magi Society, you may log onto our Members Only Website and view in advance an EXTRA MONTH of the Magi Society Love & Money Calendar. This allows our members to use the stars to plan ahead by an extra month. Members of the Magi Society may click here to begin the process of logging onto the new Members Only Website. |
| [Editor's Note: These days were chosen specifically as the best and worst days for the activities specified and you should not assume that a good day for one activity is a good day for anything and everything else. For example, a good day to get married is not a good day to get divorced. Although the good and bad dates we provide you are applicable for everyone, just how good or bad each day is will vary depending on your individual transits. For example, if you are having several Cinderella Transits on one of our good days, then the day can be fabulous for you. Or if you are having Cinderella Transits on one of our bad days, then the day will not be as bad for you as it will be for most people because your transits are better than most.] |
| FOR THE ACTIVITY LISTED BELOW: | THE BEST DAY THIS MONTH TO DO IT IS: | AND THE WORST DAY TO DO IT IS: |
| 1. The best and worst astrological days this month to get married (Make sure your transits are also good!) | January 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 2. The best and worst astrological days this month to buy something simple like a toaster or clothes | January 1, 11, 12, 14, 15, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 3. The best and worst astrological days this month to pay a bill for anything, such as rent, credit cards, utilities, department stores, etc. | January 1, 11, 12, 14, 15, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 4. The best and worst astrological days this month to sign a financial contract like a lease, tax return, car loan, etc. | January 1, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 5. The best and worst astrological days this month to make a business proposal and opening bank or brokerage accounts | January 1, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 6. The best and worst astrological days this month to deposit money into financial accounts like your bank account | January 1, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 7. The best and worst astrological days this month to ask for a promotion or raise, go on a job interview | January 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 8. The best and worst astrological days this month to meet anyone new for financial purposes like a prospective client | January 1, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 9. The best and worst astrological days this month to go out on a first date | January 1, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 10. The best and worst astrological days this month to enjoy an especially romantic evening | January 1, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 11. The best and worst astrological days this month to look for or meet with that special person who will fulfill your dreams | January 1, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 12. The best and worst astrological days this month to ask for a commitment from a loved one, such as a promise to be more understanding | January 1, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 13. The best and worst astrological days this month to ask for forgiveness and rekindle a relationship gone awry | January 1, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 14. The best and worst astrological days this month to start a new medication or begin anything new related to your health, including having any dental work done. | January 1, 11, 12, 14, 15, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 15. The best and worst astrological days this month to initiate a new contact like emailing or calling someone you do not know and laying the groundwork for making a proposal later on, or having the first meeting with someone new like a doctor, lawyer, broker, etc. | January 1, 11, 12, 14, 15, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 16. The best and worst astrological days this month to install a new APP or update an operating system on your Smart Phone. | January 1, 11, 12, 14, 15, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
| 17. The best and worst astrological days this month to start a new job. | January 1, 23, 30 | January 4, 5, 10, 13, 17, 18, 19, 26, 27, 28 |
KDO de NOEL !!!
Connaissance des temps, Éphémérides astronomiques

Cet ouvrage d’éphémérides est destiné aux astronomes, aux enseignants et aux étudiants.
Le cœur de cet ouvrage présente, pour l’année en cours, les éphémérides tabulées du Temps sidéral, des variables liées aux nouveaux paradigmes de l’Union astronomique internationale sur les systèmes de référence et les coordonnées du Soleil, de la Lune et des planètes, de Pluton, Cérès, Pallas, Junon et Vesta ; il fournit également les quantités nécessaires au calcul des positions des satellites de Mars, des satellites galiléens de Jupiter, des huit premiers satellites de Saturne et des cinq principaux satellites d’Uranus.
Un chapitre explicatif fournit les informations théoriques permettant de faire les calculs par soi-même ou d’utiliser le logiciel accompagnant l’ouvrage.
Ce volume est le 344e d’une éphéméride créée en 1679 qui a paru sans interruption depuis sa création. Ancienne par sa conception, mais toujours moderne dans sa réalisation, la version actuelle s’appuie sur une partie des récents développements méthodologiques menés à l’IMCCE.
- Voir la table des matières
- Format 17 × 24 cm – 186 pages
- Éditeur : IMCCE
- ISBN : 978-2-910015-85-5
Cet ouvrage est disponible gratuitement en version pdf : télécharger l’ouvrage. Il sera disponible très prochainement en version papier à la demande sur le site de la librairie BoD.
Un logiciel de calcul d’éphémérides accompagne l’ouvrage :
La tétrabible
 OU LES QUATRE LIVRES DES JUGEMENTS DES ASTRES
OU LES QUATRE LIVRES DES JUGEMENTS DES ASTRES
TRADUIT PAR NICOLAS BOURDIN
De Ptolémée (né vers 90 après J.-C. à Ptolémaïs d’Hermias, dans la Thébaïde d’Égypte, mort à Canope vers 170), on ne retient souvent que le système astronomique qui fait de la Terre le centre de l’Univers. Et pourtant, cinq siècles avant lui, Aristarque de Samos avait émis l’hypothèse que la terre tournait sur elle-même autour du soleil.
Mais Ptolémée est d’abord un mathématicien. Les pages de sa Syntaxe mathématique, plus connue sous le nom d’Almageste (Le grand traité), reprennent partie les données et observations d’un autre astronome alexandrin, Hipparque de Nicée. Dans son Traité d’astrologie, Claude Ptolémée nous transmet le résultat de plus de sept siècles de science grecque.
Le Tetrabiblos fut l’ouvrage le plus célèbre de l’Antiquité. Il exerça et exerce encore une grande influence dans l’étude des corps célestes de la sphère sublunaire.
De Ptolémée (né vers 90 après J.-C. à Ptolémaïs d’Hermias, dans la Thébaïde d’Égypte, mort à Canope vers 170), on ne retient souvent que le système astronomique qui fait de la Terre le centre de l’Univers. Et pourtant, cinq siècles avant lui, Aristarque de Samos avait émis l’hypothèse que la terre tournait sur elle-même autour du soleil.
Mais Ptolémée est d’abord un mathématicien. Les pages de sa Syntaxe mathématique, plus connue sous le nom d’Almageste (Le grand traité), reprennent partie les données et observations d’un autre astronome alexandrin, Hipparque de Nicée. Dans son Traité d’astrologie, Claude Ptolémée nous transmet le résultat de plus de sept siècles de science grecque.
Le Tetrabiblos fut l’ouvrage le plus célèbre de l’Antiquité. Il exerça et exerce encore une grande influence dans l’étude des corps célestes de la sphère sublunaire.
TÉLÉCHARGEZ PDF
TÉLÉCHARGEZ EPUB
POD (PRINT ON DEMAND)
Pour une impression papier,
cliquez sur l'image
Pour une impression papier,
cliquez sur l'image
Gratuit
MA VOCATION: DIFFUSER LA CONNAISSANCE.
C’est pourquoi l’Arbre d’Or existe et que je tiens à sa survie malgré la constatation sur la durée que la rentabilité du eBook est «confidentielle», euphémisme pour dire qu'elle est nulle. A l’instar de la musique et de la video, le eBook se répand gratuitement; ce qui, pour ma part, aurait plutôt tendance à me réjouir, c’est la preuve que la soif de connaissance existe toujours et j'adore l'idée qu'elle puisse être accesible à tous. C’est en raison de cette vocation que je décide de me passer de plateforme marchande et de proposer les ouvrages en téléchargement libre.
C’est pourquoi l’Arbre d’Or existe et que je tiens à sa survie malgré la constatation sur la durée que la rentabilité du eBook est «confidentielle», euphémisme pour dire qu'elle est nulle. A l’instar de la musique et de la video, le eBook se répand gratuitement; ce qui, pour ma part, aurait plutôt tendance à me réjouir, c’est la preuve que la soif de connaissance existe toujours et j'adore l'idée qu'elle puisse être accesible à tous. C’est en raison de cette vocation que je décide de me passer de plateforme marchande et de proposer les ouvrages en téléchargement libre.
MA NÉCESSITÉ: SURVIVRE FINANCIÈREMENT.
Pour cela, je dois migrer le site sur une nouvelle interface et devinez quoi? La manoeuvre n’est pas automatique. Il s'agit de recréer tous les articles et tous les liens, sans compter la nouvelle structure du site que je confie à un webmaster, certaines choses dépassant mes compétences. Tout cela a un coût en temps et en argent, c'est pourquoi cet appel aux dons.
Ils servent à arroser les racines l’Arbre d’Or et lui permettent non seulement de survivre mais évenuellement de continuer à grandir. En effet, je continue à publier les bons manuscrits qui me sont proposés, mais uniquement à compte d'auteur. Contactez-moi pour en connaître les conditions.
Téléchargez tout ce qui vous plaît,
Faites (ou ne faites pas) un don selon vos moyens,
Faites (ou ne faites pas) un don selon votre humeur,
Manifestez votre satisfaction,
Exprimez vos souhaits,
...Savourez votre lecture.
Pour cela, je dois migrer le site sur une nouvelle interface et devinez quoi? La manoeuvre n’est pas automatique. Il s'agit de recréer tous les articles et tous les liens, sans compter la nouvelle structure du site que je confie à un webmaster, certaines choses dépassant mes compétences. Tout cela a un coût en temps et en argent, c'est pourquoi cet appel aux dons.
Ils servent à arroser les racines l’Arbre d’Or et lui permettent non seulement de survivre mais évenuellement de continuer à grandir. En effet, je continue à publier les bons manuscrits qui me sont proposés, mais uniquement à compte d'auteur. Contactez-moi pour en connaître les conditions.
Téléchargez tout ce qui vous plaît,
Faites (ou ne faites pas) un don selon vos moyens,
Faites (ou ne faites pas) un don selon votre humeur,
Manifestez votre satisfaction,
Exprimez vos souhaits,
...Savourez votre lecture.
Calendrier Lunaire Janvier 2022
En ce début d’année, un aperçu des différentes applications
Du Calendrier Lunaire 2022
La viniculture et la Lune
Pour ne pas stopper l’activité du vin, soutirez par exemple entre le 3 et le 15 (en évitant le 7, le 11 et le 13) .
Pour ne pas stopper l’activité du vin, soutirez par exemple entre le 3 et le 15 (en évitant le 7, le 11 et le 13) .
Réduire l’utilisation des insecticides avec la Lune
Certains aspects planétaires vont renforcer l’action des produits, et donc permettre de diminuer les doses :
Appliquez les produits le matin ou le soir du 22 au 26.
Certains aspects planétaires vont renforcer l’action des produits, et donc permettre de diminuer les doses :
Appliquez les produits le matin ou le soir du 22 au 26.
Pour un meilleur compost avec la Lune
Epandage du fumier ou compostage de surface, l’assimilation sera plus rapide le 23, 24 ou 25.
Epandage du fumier ou compostage de surface, l’assimilation sera plus rapide le 23, 24 ou 25.
Couper les bois en bonne Lune
Abattage des bois destinés aux charpentes entre le 18 et le 29 (en évitant le 27 matin).
Abattage des bois destinés aux charpentes entre le 18 et le 29 (en évitant le 27 matin).
Les animaux et la Lune
Traitez contre les vers le 23 ou le 24.
Traitez contre les vers le 23 ou le 24.
Faire son pain avec la Lune
Entre le 3 et le 15 vous obtiendrez un pain au levain bien levé et plus savoureux.
Entre le 3 et le 15 vous obtiendrez un pain au levain bien levé et plus savoureux.
Notre bien être avec la Lune
Pour une épilation plus efficace nous vous conseillons la période du 18 au 29.
Le jardin est au repos c’est le moment de choisir ses graines
et de prévoir le plan pour la saison prochaine.
Pour une épilation plus efficace nous vous conseillons la période du 18 au 29.
Le jardin est au repos c’est le moment de choisir ses graines
et de prévoir le plan pour la saison prochaine.
Re:
Re:
Re:
Re:
Re:
Re:
Re:
Re: |
Re:
Re:
Re:
Re:
Re:
Re:
Re:
Re: |
Re:
Re:
Re:
Re:
Re:
Re:
Re:
Re: |
Re:
Re:
Re:
Re:
Re:
Re:
Re: |
Class action against Sanofi wins French court backing
Jan 05 2022

The court said Sanofi was 'at fault' for not warning pregnant patients early enough
Paris (AFP) - A French court on Wednesday allowed a class-action lawsuit to go ahead against pharmaceutical giant Sanofi by families of victims of Depakine, an epilepsy drug that can severely damage foetal development.
Depakine, a valproic acid drug sold since 1967 sometimes under different brand names, marked great progress in the treatment of epilepsy, and of manic phases for bipolar patients.
But valproic acid is also believed to have caused malformations for between 2,150 and 4,100 children since it was launched in 1967, and neuro-development problems for between 16,600 and 30,400 children, according to French health authorities.
A class action initiated in 2017 by the Apesac association alleged that Sanofi did not inform patients early enough about the risks of birth defects and slowed development in children whose mothers were prescribed the drug during pregnancy.
In Wednesday’s verdict the court ruled that the lawsuit was “valid” and could proceed, saying Sanofi “was at fault because it failed to meet the obligation for vigilance and the obligation to inform” concerning the risks of Depakine for the foetus.
Sanofi said in a statement that it had always been “transparent by alerting the health authorities”, and that it would appeal the verdict.
With the ruling the court opened the door to France’s first class-action suit in the health sector, following a 2016 change in the law which previously allowed such action only against consumer goods companies.
Sanofi had “produced and marketed a defective product”, it said.
Based on medical knowledge at the time, Sanofi should have warned patients about congenital malformations from 1998 onwards and about neuro-developmental issues – which were less well understood previously – from 2001.
Sanofi only started issuing such warnings clearly in 2006, advising doctors not to prescribe Depakine to female patients, except those unable to get pregnant or with an intolerance or non-reaction to all alternative treatments.
A lawyer for the Apesac association, Charles Joseph-Oudin, told AFP that the court’s verdict brought “great relief” to the families because it “recognises that Sanofi was at fault”.
The court ordered the launch of a wide-ranging campaign to inform patients and their children of the possibility of joining the class action within five years.
Last year, an administrative court, which deals with wrongdoing by officials, ruled that the French state carried some responsibility in the scandal – along with Sanofi and prescribing doctors – and ordered the authorities to pay damages to several families with severely handicapped children.
Sanofi and France’s medicines safety agency ANSM were charged with involuntary manslaughter in 2020.
Agence France-Presse
AFP journalists cover wars, conflicts, politics, science, health, the environment, technology, fashion, entertainment, the offbeat, sports and a whole lot more in text, photographs, video, graphics and online.
© 2022 AFP


 3:38
3:38 7:27
7:27 9:15
9:15
 0:02
0:02 0:02
0:02 8:44
8:44 19:33
19:33 19:33
19:33 20:22
20:22 9:46
9:46 16:50
16:50 23:43
23:43 10:33
10:33 13:36
13:36 1:16
1:16 8:36
8:36 11:31
11:31 7:07
7:07 11:51
11:51 6:25
6:25 6:25
6:25 12:08
12:08 6:31
6:31 12:24
12:24 19:11
19:11 7:21
7:21 12:40
12:40 15:46
15:46 8:48
8:48 12:57
12:57 10:42
10:42 13:17
13:17 4:07
4:07 12:51
12:51 13:41
13:41 10:24
10:24 14:11
14:11 15:04
15:04 14:49
14:49 17:10
17:10 17:10
17:10 15:37
15:37 19:02
19:02 16:35
16:35 20:36
20:36 0:48
0:48 5:02
5:02 17:40
17:40 21:49
21:49 18:50
18:50 22:40
22:40 15:02
15:02 20:02
20:02 23:09
23:09 21:15
21:15 23:16
23:16 22:27
22:27 23:02
23:02 23:02
23:02 22:24
22:24 23:41
23:41 21:23
21:23 0:57
0:57 4:56
4:56 14:40
14:40 19:56
19:56 2:16
2:16 18:03
18:03 3:38
3:38 8:34
8:34 15:45
15:45 5:01
5:01 6:20
6:20